We Finally Have Rapid At-Home Covid-19 Tests. What Happens Now?
(Posted on Thursday, November 19, 2020)

TOLEDO, OHIO, UNITED STATES – 2020/11/14: NHA COVID-19 testing sign seen in front of the Greater Grace Christian Church on Monroe St. Neighbourhood Health Association (NHA) partners with Greater Grace Christian Church (GGCC) in the Auburn-Delaware neighbourhood of Toledo, Ohio, to give residents of the area access to COVID-19 lab testing and access to the churchs food pantry. NHA provided over 100 COVID-19 tests to the community, and GGCC allowed residents to leave with baskets full of groceries. (Photo by Stephen Zenner/SOPA Images/LightRocket via Getty Images)
SOPA IMAGES/LIGHTROCKET VIA GETTY IMAGES
The Food and Drug Administration has authorized the first rapid at-home test for Covid-19. It is a critical step forward in our efforts to contain the spread of disease, though the journey is far from complete.
The test, manufactured by Lucira Health, includes a nasal swab, sample vial, and a battery-operated testing device. Users swab each of their nostrils five times then stir the swab into the sample vial before pressing it into the testing unit. After 30 minutes, the testing device will shine green and indicate either a positive or negative test result. It is the first of its kind in the United States, and the only test that lets people swab themselves, analyze the sample, and receive results entirely from home.
Public health leaders have long advocated for rapid at-home diagnostic kits to help stop the spread of Covid-19. If people suspect they’ve been infected and can quickly and easily confirm their suspicions, they can immediately self-isolate and warn those around them of their exposure, curtailing the ability of the virus to spread. Computer simulations have suggested that if Americans were tested every one to three days, transmission could be reduced by more than 80% — enough to conceivably put a rapid end to the epidemic.
This test, though welcome, doesn’t quite get us there.
First, the test is only available with a prescription. This means people will still need to jump through a few hoops — speaking with their doctor, securing a prescription, and getting the kit from the pharmacy to their home — all before they can take the test. Not only does this take time, it also opens up new opportunities for spreading the virus if the person potentially infected has to leave home. The test is also not approved for at-home use for children under age 14, so a child exposed at school would still need to visit their doctor to find out if they’re infected.
Second, the test doesn’t come cheap. According to Lucira, the test will cost around $50 US — a figure high enough to give most Americans pause and to prevent some from purchasing the test at all if payment is coming out of pocket. For rapid at-home tests to be useful at containing and potentially ending the pandemic they must be easily affordable for insured and uninsured alike, as well as widely available and regularly used.
Some have called out the accuracy of the tests — or lack thereof — as a possible cause for concern. The reality is that even tests that deliver correct results 94 or 95% of the time, as the Lucira tests claim to, are accurate enough to get the job done. While some may give a false positive or negative, at this level of accuracy home tests will still catch not just the vast majority of those infected, but the vast majority of those with an elevated viral load who may be highly contagious (see Figure 1). If everyone is tested at least twice a week, whether at homes, schools or in the workplace, many false negative results will be corrected in a subsequent test. Those testing positive could test themselves again to ensure the accuracy of their results.
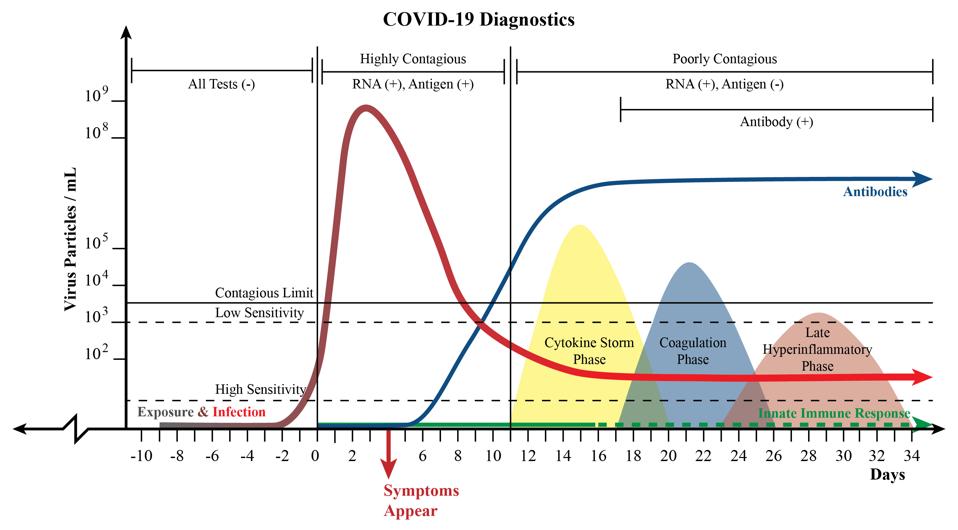
Figure 1: A chart illustrating a theoretical representation of Covid-19 disease progression. The red line represents viral load, or the amount of virus present in the body at a given point in time. The colored, rounded nodes represent advanced stages
AUTHOR
To implement twice a week testing, ideally testing the entire US population every three to four days, we would need 100 million tests every day for at least six months to wrestle the pandemic under control. The tests should cost no more than 50 cents each, a price tag that some may consider unattainable but that recent history shows is possible. Abbott Laboratories, the American pharmaceutical company, recently supplied Egypt with enough 50 cent rapid hepatitis C tests to screen their entire population of about 60 million people, helping the country effectively eliminate the disease.
Meanwhile in the United Kingdom, the Financial Times has reported that the Department of Health is purchasing one billion pounds worth of rapid tests to implement a mass testing program to control their national epidemic. The cost of each test hasn’t been publicly announced, but it’s rumored to be under $10 US per test. Not quite 50 cents but certainly much better than the fifty dollar price tag for Lucira’s device.
Once issues around cost and widespread distribution are resolved, one critical step remains: encouraging those who test positive to isolate immediately. This can’t be done through public messaging alone. Americans who test positive should be encouraged to isolate for at least fourteen days following the time of their most recent positive test. They should be able to do so without risk to their livelihoods and with additional financial support to cover any lost income or added costs for food, shelter, or medical supplies. By my calculations, this would come to about $500 per family per day. To receive funding, confirmation of infection from a healthcare provider would be required and monitoring to ensure compliance with self-isolation would be encouraged.
If Americans knew that they had an easy and affordable way to test themselves for infection and knew they would be supported financially for choosing to stay home and self-isolate to avoid infecting others, I believe nearly every American would choose to do the right thing for themselves and those around them. Some may argue the costs of a program such as this would be too high. But compared to the trillions of dollars we risk losing with more widespread lockdowns, on top of the trillions we have lost to date, the cost is a relative drop in the bucket.
Originally published on Forbes (November 19, 2020)

