New Study Using Live Virus Explores Whether Pfizer-BioNTech Vaccine Protects Against Variants
(Posted on Thursday, March 11, 2021)

SCHWAZ, AUSTRIA – MARCH 11: A nurse fills syringes with the Pfizer-BioNTech vaccine during a mass vaccination drive at SZentrum on March 11, 2021 in Schwaz, Austria. Over the next five days authorities will offer the vaccine to all eligible adults in
GETTY IMAGES
When new variants of SARS-CoV-2, the virus that causes Covid-19, were discovered to be circulating around the world, the first question for many was whether they would present a serious problem for nascent vaccination programs against Covid-19. While the answer still eludes us, study by study researchers are firming up their notions of what the future, now that it involves a virus that evolves far more rapidly than previously understood, will hold.
This early on, the general consensus is mixed. Many are optimistic that the technologies at our disposal—the same that did, after all, miraculously produce safe and efficacious vaccines in less than a year—can adapt and ultimately prevail over the new variants, which have been found to be more transmissible, immune-evasive, and in some cases more virulent than their predecessors. But several experts are also expressing caution insofar as the current generation of vaccines are concerned, in part due to recent reports that antibodies, whether collected from recovered Covid-19 patients or people recently immunized, are less effective at neutralizing artificially mutated versions of SARS-CoV-2. Moving forward, the trick will be striking a balance between these two observations: acknowledging and celebrating the good news that comes our way, but remaining vigilant and ready to take action should viral variation have longer-term consequences on our ability to contain the pandemic this year and for years to come.
A correspondence published this week in the New England Journal of Medicine gives us another reason to be of both minds. It details a study of 20 samples of serum that were taken from 15 participants who received two doses of the Pfizer-BioNTech vaccine, then exposed to five viruses carrying mutations seen in the B.1.1.7, B.1.351, and P.1 lineages of the virus—variants that originated in Britain, South Africa, and Brazil, respectively. That they used live viruses is significant, since previous research relied instead on pseudotyped viruses created in-lab by decorating the envelope of other viruses with SARS-CoV-2 spike proteins.
The serum, collected two to four weeks after the second immunization, was able to neutralize all five, though the virus carrying a more complete subset of mutations from the B.1.351 variant proved more resistant than the rest, a finding consistent with previous studies of its potential for immune evasion (figure 1). Many media reports have interpreted this as confirmation that two doses of the Pfizer-BioNTech vaccine is adequate protection against the new variants. While the data is certainly encouraging, a number of caveats that weren’t fully addressed in the publication are worth mentioning.
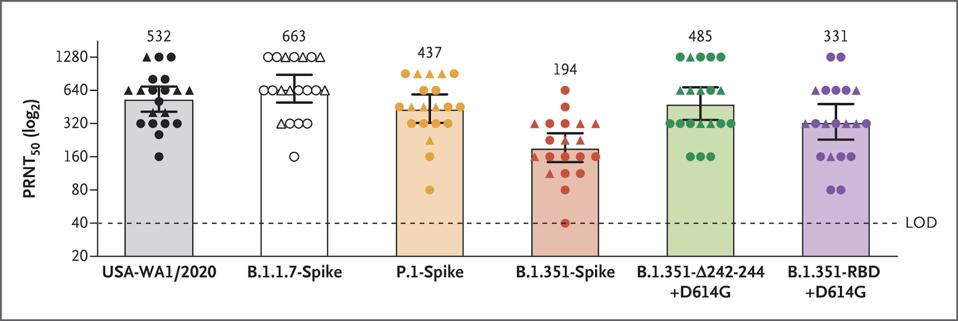
Figure 1. Serum Neutralization of Variant Strains of SARS-CoV-2 after the Second Dose of BNT162b2 Vaccine.
“NEUTRALIZING ACTIVITY OF BNT162B2-ELICITED SERUM” HTTPS://WWW.NEJM.ORG/DOI/FULL/10.1056/NEJMC2102017#ARTICLE_CITING_ARTICLES
First, infection in a test tube is not the same as infection in a person. The researchers who conducted the study acknowledged this, citing a need for “real-world evidence” to further corroborate their findings, but don’t elaborate beyond that. The plaque reduction assay they used in their experiments may not necessarily mimic the full potential and limitations of the antibody response in a person undergoing infection. For a bigger, more accurate picture, cell cultures, primate models, and other methods must be used.
Second, the scope of the study doesn’t go beyond mutations that are part of the spike protein, even though this region comprises only 10 percent of the entire viral genome (see difference between Figure 2, a linear representation of the mutations used in one virus looked at in this study, compared to Figure 3, a linear representation of the P.1 genome vs. the P.1 spike). The mutations that weren’t included, which appear in regions like the virus’s non-structural and accessory proteins, may play a pivotal role in affecting antigenicity and immunogenicity that we’ve simply yet to understand. Why these mutations have been neglected so consistently is questionable; if nature is sending us a message, we’d do best to listen in. Future studies must aim to be more comprehensive or risk creating blind spots in the development of variant-specific drugs and vaccines.
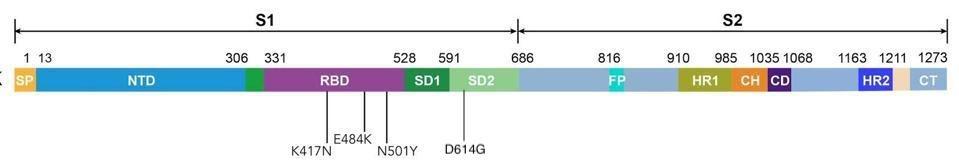
Figure 2. Linear representation of the spike protein carrying mutations that the researchers studied: K417N, E484K, N501Y, and D614G.
AUTHOR
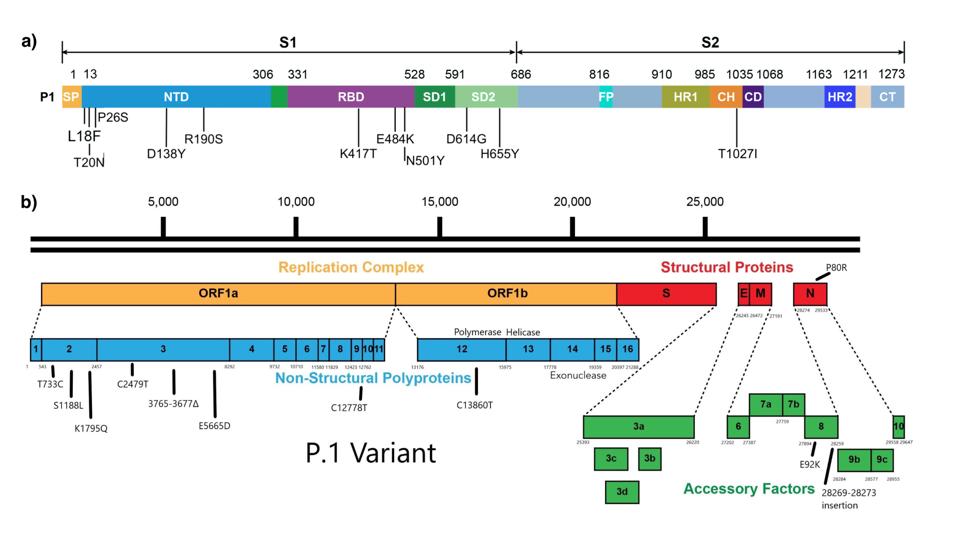
Figure 3. (A) Linear representation of the spike protein of the P.1. variant. (B) Representation of the broader genome of the P.1 variant, including mutations in the nonstructural proteins and accessory factors.
AUTHOR
Third, the serum samples were collected from vaccinees when neutralizing activity was at its theoretical peak. Several studies, including a far more extensive one published in Nature last month and included six recipients of the Pfizer-BioNTech vaccine as well as 14 who were administered the Moderna vaccine instead, show that the potency of sera, whether it belongs to a recovered patient or vaccinee, drops considerably over time (Figure 4). This is true of samples exposed to the original SARS-CoV-2 strain that originated in Wuhan, but even more so of those exposed to new variants.
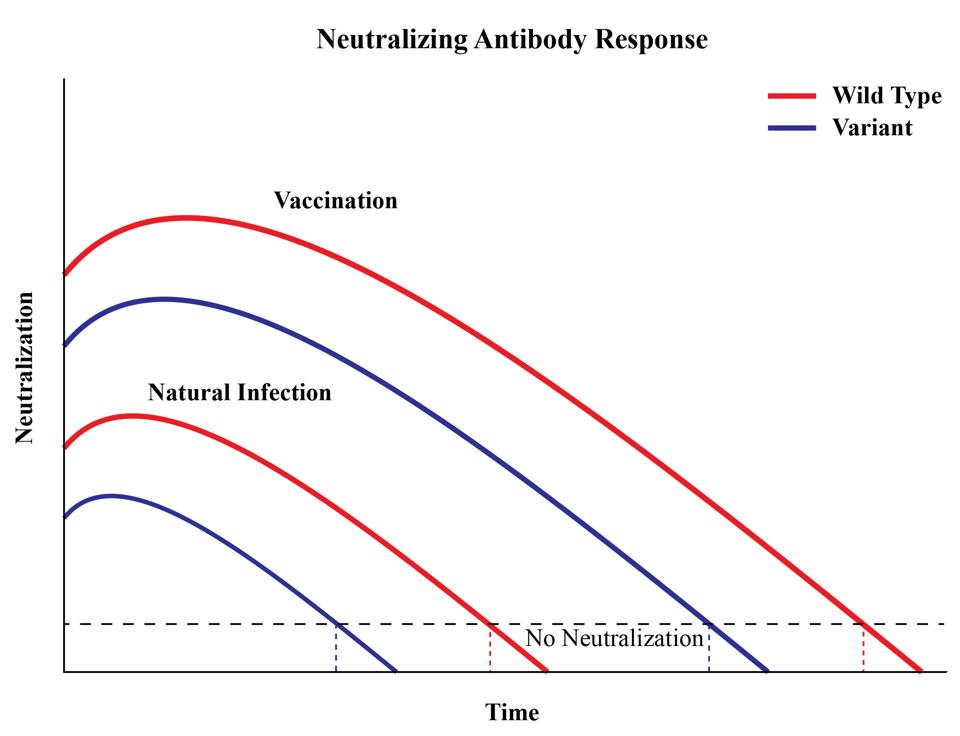
Figure 4. A representation of antibody titers over time.
AUTHOR
A clinical study conducted by Pfizer and BioNtech is already underway that will test the efficacy of a recently developed booster shot against the new variants. Ideally this study and others like it will involve the full breadth of the viral genomes being analyzed, rather than a narrower selection, in addition to a longer timeline that accounts for the potential reduction in neutralizing antibodies. For now, let’s remain hopeful that more promising evidence awaits us, but keep in mind that SARS-CoV-2 is a force of nature to be reckoned with—one that has surprised us several times over, and may do so again if we’re not sufficiently careful.
Originally published on Forbes (March 11, 2021)

