Preventing Fecal-Oral And Fecal-Aerosol Transmission Of Covid-19
(Posted on Monday, March 22, 2021)
This is the first in a series of articles on the neglected pathologies and transmission routes of Covid-19.

HONG KONG, CHINA – JANUARY 20: Workers check the sewage system where COVID-19 cases have been confirmed in the Jordan district on January 19, 2021 in Hong Kong, China. Hong Kong issues an isolation order for entire residential blocks for the first
GETTY IMAGES
Last year, I conducted an interview with a friend who traveled to Shanghai and was forced to quarantine in a hotel for 11 days under the supervision of local health authorities. He was brought hot meals, subjected to temperature checks twice daily, and charged not a cent for his stay—standard protocol in China and other countries that, prior to the widespread imposition of travel bans, isolated and accommodated visitors from abroad early on in the Covid-19 pandemic.
One detail, however, struck me as rather curious, if not startling. When my friend checked in, a woman handed them a blue bucket and a little bottle of disinfectant tablets. “For toilet,” she told him, then laughed when she saw the look of shock and horror on his face. “No, no, no, no. Not for that. Dissolve the tablets in water in the bucket, then dump the mixture into the toilet before you flush.” It didn’t matter whether it was number one or number two, my friend told me. He had to treat his waste as if it was potentially infectious and carrying the virus that causes Covid-19, SARS-CoV-2—using half a bucket of water and six tablets for urine, or a whole bucket and twelve tablets for feces.
Disinfecting toilets isn’t the only safety measure that addresses the possibility that SARS-CoV-2 can be transmitted through human waste. More recently, China has been heavily criticized, particularly by the Japanese government, for using anal swabs to test incoming travelers for Covid-19 either instead of or in addition to the traditional nasopharyngeal swabs. These interventions, like most China has implemented to contain further spread, may be extreme, but the rationale behind them isn’t. Evidence not just from the past year, but the original SARS pandemic, shows that SARS-CoV-2 infects the intestines and colon and from there spreads to others, traveling from the toilet to the sewer to the water we use and the air we breathe—one reason why sewage surveillance across cities and within neighborhoods has become so commonplace.
A 3D visual depiction of the gut.
ALPHA TAURI 3D GRAPHICS / SHUTTERSTOCKPathogenesis and severity: SARS-CoV-2 in the gut
While it’s true that respiratory symptoms are far and away the most ubiquitous sign of Covid-19, the prevalence of gastrointestinal symptoms is often underestimated. Diarrhea, which occurs in 20 to 25 percent of Covid-19 patients, is the most common, but nausea, vomiting, and abdominal pain are reported with relative frequency as well. More serious symptoms, like acid reflux, gastrointestinal bleeding, constipation, and hematochezia (also known as blood in stool), are a rarity, but not so rare as to be total outliers.
Other respiratory viruses, like influenza and even SARS-CoV, also cause their share of gastrointestinal symptoms, though why exactly this is the case isn’t certain. Diarrhea, for example, is a symptom shared by SARS, MERS, and Covid-19. Early studies of SARS-CoV-2 speculated that the virus could infect organs beyond the lungs, a theory corroborated by experiments involving organoids that showed how it could spread to the bloodstream and the kidneys. Using gut organoids, one such experiment found SARS-CoV-2 to be capable of replicating in the intestines, supporting the notion that the virus not only reaches our gut tissues, but damages them as well—hence the gastrointestinal discomfort many Covid-19 patients experience. But organoid experiments are ultimately limited in their ability to simulate virus-host interactions, offering insight into only one system at a time, rather than the complex whole.
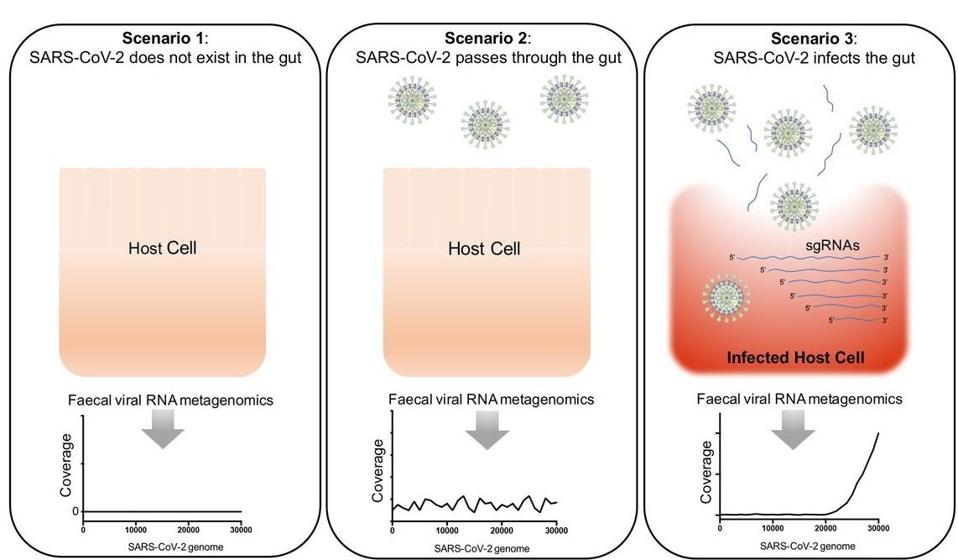
Figure 2. Three scenarios hypothesised for the presence and infectivity of SARS-CoV-2 in the gut of patients with COVID-19, and the detection of SARS-CoV-2 virus by faecal viral RNA metegenomics sequencing.
“DEPICTING SARS-COV-2 FAECAL VIRAL ACTIVITY IN ASSOCIATION WITH GUT MICROBIOTA COMPOSITION IN PATIENTS WITH COVID-19” HTTPS://GUT.BMJ.COM/CONTENT/70/2/276A more recent study, published in Nature last month, looked across the existing literature on gastrointestinal symptoms and attempted to extrapolate their causal mechanism, though this proved to be difficult. For diarrhea alone the possible explanations ranged widely. It could be osmotic diarrhea caused by an inflammatory immune response that extends to the gut. It could also be more indirect than that, caused by changes to the gut biome—more specifically, its bacterial composition—that trace back to inflammation of the lungs, rather than the gut itself. One question that has beleaguered scientists and doctors almost since the pandemic began is why the virus persists so long in some Covid-19 patients and not others. I speculate that infection and damage to the gut might have a part to play in such cases of persistence, in which case a symptom like diarrhea or gastrointestinal bleeding would represent only the tip of the iceberg of more extensive damage.
Were researchers to conduct studies that deepen our understanding of the gastrointestinal symptoms triggered by Covid-19, doctors would be better able to treat them in clinical settings. This raises another question—whether or not manifestation of these symptoms can be linked to the severity of disease. At least one study, published in December 2020, has concluded based on multivariate analysis that this might be the case, though other studies found no statistically significant correlation.
A more explicit yet less obvious link may exist, however, between critical cases of Coivid-19 and interleukin 18 (IL-18), an inflammatory cytokine that appears primarily in intestinal cells and plays a critical role in lung diseases like asthma chronic obstructive pulmonary disease. When an overreaction of the immune system known as a cytokine storm occurs in someone sick with Covid-19, it can lead to the onset of severe pneumonia or death. Studies show that the fecal samples of people who test positive for Covid-19 have higher IL-18 levels than those who don’t. Based on this data, the authors of the February Nature study propose that elevated levels IL-18 might not just indicate gut infection by SARS-CoV-2, but hint at the possibility of an incoming cytokine storm, too. If their conjecture is correct, drugs that block IL-18 might be of use to doctors treating critically ill Covid-19 patients.
In any case, the information we currently have on hand isn’t enough to act on. More research remains to be done on the gastrointestinal tract if we’re to unravel its pathogenic implications in their entirety—not just for Covid-19 patients with severe symptoms, but pediatric patients as well. Some children who have contracted the virus and have also developed a rare inflammatory condition known as MIS-C that, in some cases, inflames the gut.
Fecal-oral and fecal-aerosol transmission of Covid-19
Filling the gaps in our knowledge around gastrointestinal symptoms and the severity of potential Covid-19 gut infections, as I’ve discussed, can do more than improve how we treat our ill. Recall the story of my friend with the bucket and disinfectant tablets in Shanghai. If SARS-CoV-2 does indeed replicate in the intestines, it may be able to exit the body intact, contaminating our waste—meaning we’d have to shift our public health strategies accordingly. Though the main way Covid-19 spreads will always be from person to person, exposure to infectious waste and sewage has a part to play in starting outbreaks, especially in apartment buildings or schools where many people share close quarters at regular intervals of the day.
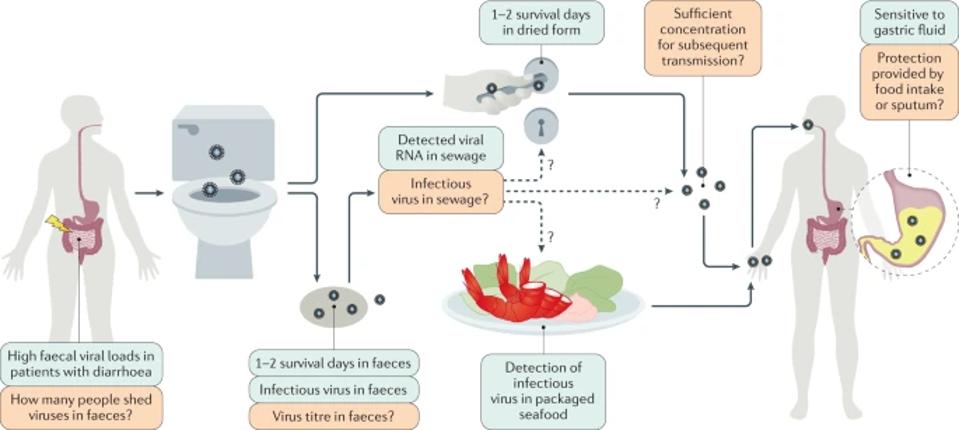
Figure 3. The exact faecal–oral transmission route is not yet established for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); green boxes show confirmed findings, whereas orange boxes depict open questions.
“POTENTIAL INTESTINAL INFECTION AND FAECAL–ORAL TRANSMISSION OF SARS-COV-2” HTTPS://WWW.NATURE.COM/ARTICLES/S41575-021-00416-6In theory, the virus can travel from one person’s waste to another’s lungs through one of two routes. The first is fecal-oral route, which involves either direct ingestion of contaminated water through the mouth or indirect exposure through the eyes or nose. The second is what I call the fecal-aerosol route, whereby the virus enters the body via sewer gas or even smaller, yet still virus-laden particles known as aerosols. The ability of the virus to successfully traverse either depends on whether it can remain infectious and replicate at sufficient concentrations as it slogs through the digestive juices of the human gut.
There is some virological evidence that SARS-CoV-2 can, in fact, replicate in the gut and shed through feces. One study, conducted in Zhejiang, China, discovered viral RNA in the stool of 59 percent of Covid-19 patients tested, remaining at detectable levels for three weeks on average. Another study found that the virus persisted longer in fecal samples than even respiratory samples. Though the presence of viral RNA doesn’t necessarily indicate the presence of live, actively replicating virus, the latter has been isolated and cultured from fecal samples in the laboratory.
No coincidence is it that the expression of ACE-2, the receptor SARS-CoV-2 uses to attach to human cells, is at its highest in the small intestine’s enterocytes, or the cells that line its inner surface. On a separate but related note, ACE-2 is also amply expressed in the vagina and uterus, alluding to the potential for sexual transmission—which may go without saying, but must be accounted for nevertheless.
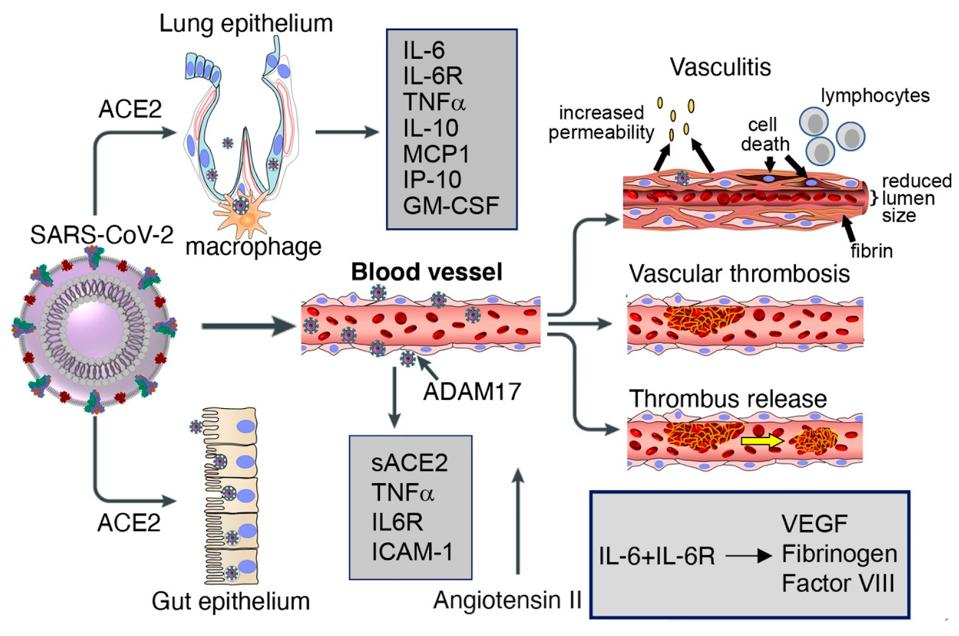
Figure 4. Lung and gut epithelia, macrophages, and vascular endothelia are infected by SARS-CoV-2 in COVID-19.
“VASCULOPATHY AND COAGULOPATHY ASSOCIATED WITH SARS-COV-2 INFECTION” HTTPS://WWW.MDPI.COM/2073-4409/9/7/1583/HTMFecal-aerosol transmission, according to a research paper published in Annals of Internal Medicine in December 2020, was suspected to be the cause of a Covid-19 outbreak in a high-rise apartment building in Guangzhou, China that infected at least nine people across three separate households. Prior to the outbreak, one of the families had traveled to Wuhan, the original epicenter of the pandemic. The other two had not, nor did they have any close contact with the first family in the weeks leading up to symptom onset. In addition to swabbing the infected residents, researchers who investigated the case collected environmental and air samples from their private residences and common areas, including elevators and ventilation outlets on the roof. Almost all of the environmental samples that came back positive were from the master bathrooms, giving the researchers reason to believe that the drainage pipes connecting the three units were to blame.
If fecal-aerosol transmission was the source of the Guangzhou outbreak, it wouldn’t be without precedent. In 2003, a much larger outbreak of SARS, the first human coronavirus pandemic, infected more than 300 people living in Amoy Gardens, a large apartment complex of about 15,000 residents in Hong Kong. The team of experts dispatched to contain the spread, which happened virtually overnight and ultimately killed 42 people, traced it back to two causal factors. The first was the index patient, or “patient zero,” a 33-year-old man who actually lived in Shenzhen but frequented his brother’s Amoy Gardens apartment and, once he got sick with SARS, had explosive diarrhea. The second was the complex’s defective drainage system, which dried up the toilets of many residents—most of them in Block E, the building that ultimately had the most deaths—forcing them to resort to bucket flushing. Exhaust fans installed in the bathrooms for ventilation purposes may have unwittingly contributed to the spread of viral particles as well.
Looking over this wide-ranging body of evidence, it becomes clear that our lack of understanding of how SARS-CoV-2 interacts with the human gut and transmits itself through human waste isn’t due to their unimportance. Much as we all want this pandemic to be behind us, expanding and deepening our knowledge of the virus that caused it is the only way we can prevent it from happening again. That means moving beyond the general assumptions that have guided us up until this point and making those less obvious connections that can firm up any protection we’ve managed to acquire.
It’s time for public health officials to mount a more comprehensive response to potential fecal-oral and fecal-aerosol transmission of Covid-19. Official guidance should be issued that recommends anyone infected with Covid-19 to to disinfect their feces, with extra emphasis on implementation in congregate living situations like prisons, long-term care centers, and military installations. The same set of guidelines should advise people living in apartment buildings or complexes to notify their landlords or local authorities if they smell sewer gas—the most common point of entry being dried-out drain traps in the sink, bath, or shower. Finally, if any of us smell sewer gas while walking down the street, we should take it upon ourselves to notify local authorities who can take remedial action and nip the problem in the bud.
We can’t make the mistake of allowing our public health responses to grow stagnant as the virus continues to evolve. After all, what is the point of acquiring more knowledge on SARS-CoV-2 if we can’t use it to protect ourselves and others?
Originally published on Forbes (March 22, 2021)

