Discovery Of A Novel Monoclonal Antibody That Neutralizes A Broad Range Of Coronaviruses
(Posted on Wednesday, May 19, 2021)
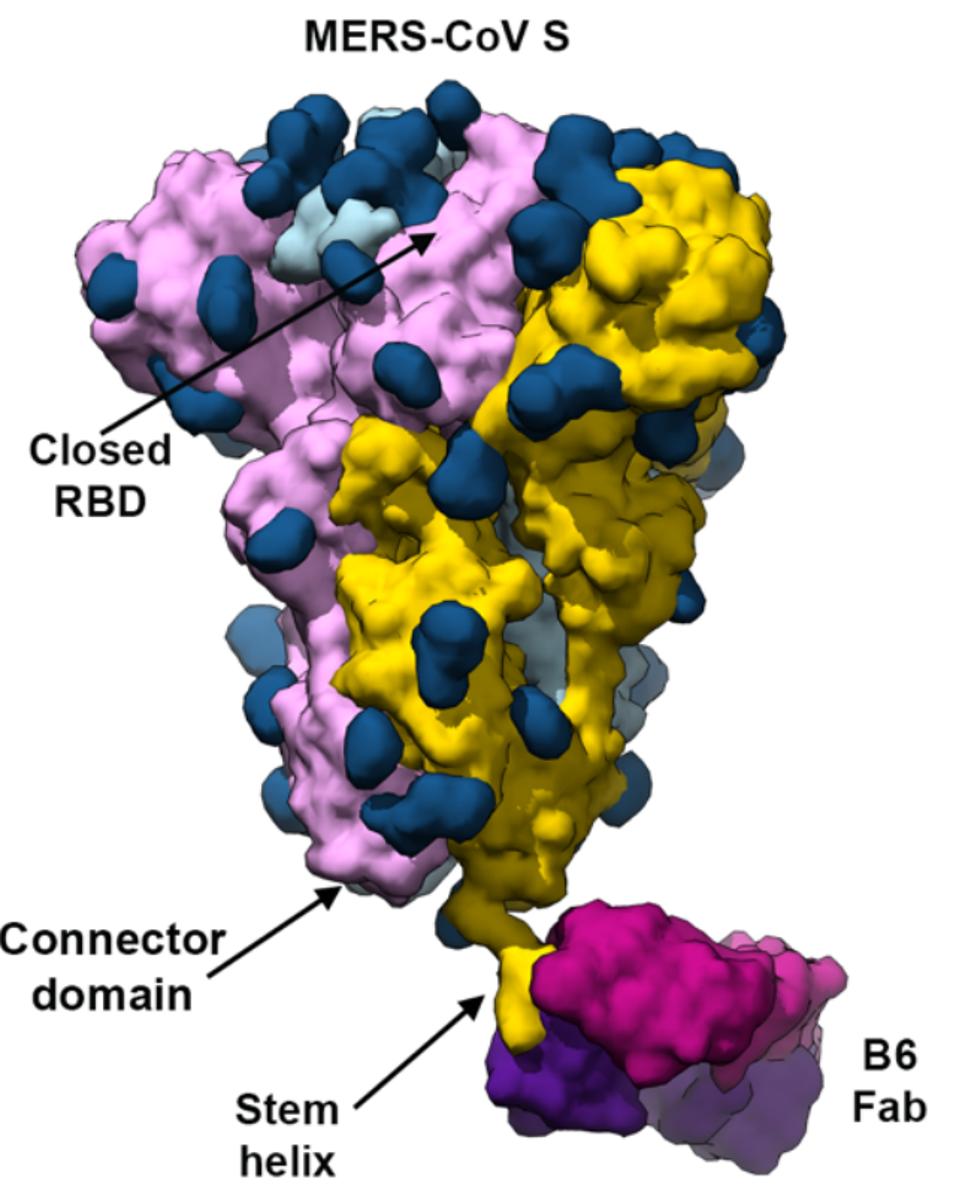
B6 binding to the MERS-CoV-2 spike
SAUER ET AL
The Covid-19 pandemic is no isolated incident. Coronaviruses have been coming after us for years. In the past 60 years, there have been as many as five seasonal cold-causing coronaviruses. In the past 20 years, we have dealt with three lethal coronaviruses: SARS-CoV, MERS-CoV, and SARS-CoV-2, the last of which has conservatively killed 3.5 million people and counting.
The SARS-CoV-2 virus belongs to the B sarbecovirus clade, along with SARS-CoV. There are also C clade viruses, including MERS-CoV, and A clade viruses, which include many cold-causing coronaviruses. As we search for treatments for SARS-CoV-2, the question is raised: can we find measures to treat all broad classes of coronavirus unilaterally?
This search has already begun and one approach of note is monoclonal antibodies. These antibodies have been found to effectively treat and prevent early infection when used as a therapy. Within monoclonal antibodies, researchers aim to find those which potently neutralize not only SARS-CoV-2 but also SARS-CoV and viruses from the A and C clades as well.
In a study by Sauer et al., researchers aimed to find a broadly neutralizing monoclonal antibody not in human convalescent sera, but in that of mice. To do this, they inoculated the mice twice with a stabilized MERS-CoV spike trimer and once with a stabilized SARS-CoV-2 spike trimer. Their aim was to locate antibodies that broadly combated both viruses.
The researchers gathered Fab fragments from the vaccinated mice and connected them to human Fc receptors. After introducing the antibodies to the different coronaviruses, they selected one antibody, B6, which strongly bound to A and C clade viruses, but not for B clade viruses (Figure 1). Additionally, B6 was only able to potently neutralize A and C clade viruses, namely MERS-CoV and cold-causing coronaviruses. SARS-CoV and SARS-CoV-2 were left unneutralized (Figure 2).
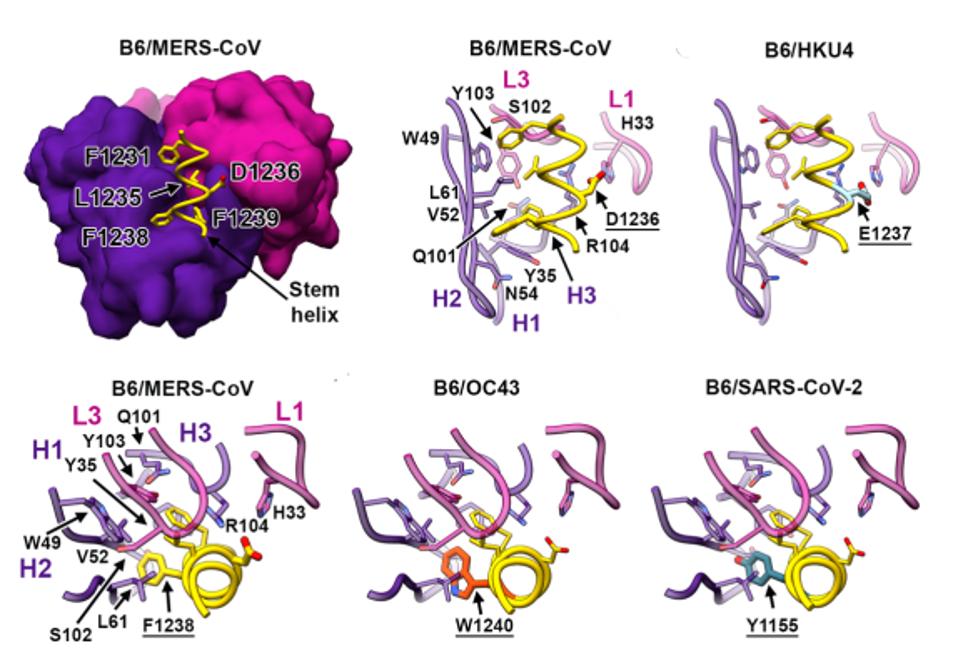
FIGURE 1: B6 binding at the amino acid level.
SAUER ET AL

FIGURE 2: B6 neutralization of MERS-CoV, SARS-CoV, and SARS-CoV-2.
SAUER ET AL
It seems, however, that the B6 antibody is capable of binding in SARS-CoV and SARS-CoV-2 to what the researchers label a cryptic epitope. Why, then, does the binding and neutralization fall off? To examine this, the researchers used cryo-electron microscopy to see exactly where the antibody was binding. In A and C clade viruses, the cryo visual of the region to which the antibody bound was clear (Figure 3). In SARS-CoV and SARS-CoV-2, however, the binding was disordered. This is surprising as this is usually a highly ordered area. They investigated the hidden binding by taking higher resolution pictures of the unusual binding between B6 and SARS-CoV-2.
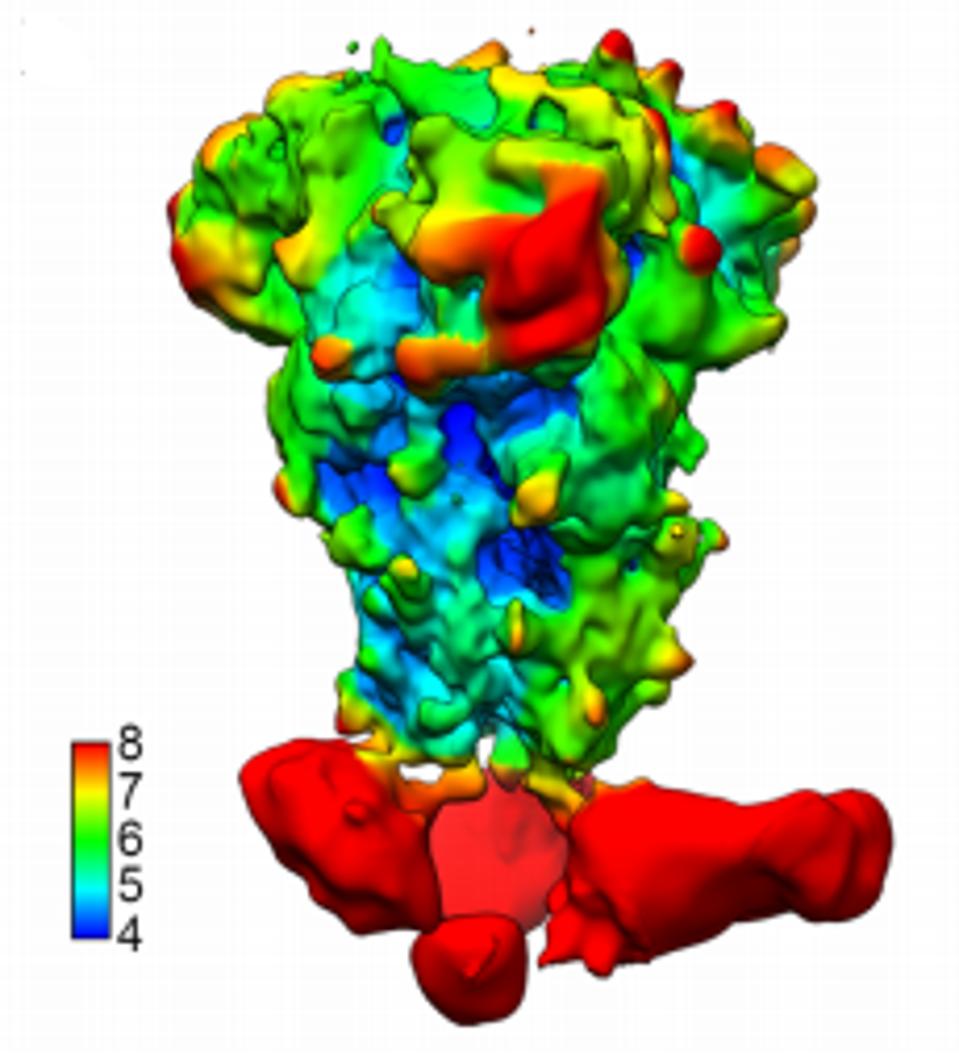
FIGURE 3: Cryo-electron microscopy of B6 binding to MERS-CoV.
SAUER ET AL
Testing individual peptide fragments across the general region of binding noted in other viruses, the researchers found their clearer picture. It seemed that in A and C clade viruses, the trimer is less tightly bound than in B clade viruses. The looser trimer stem helix bundle in MERS-CoV and cold-causing coronaviruses was unraveled during binding, allowing a clearer picture during cryo-electron microscopy (Figure 4). In SARS-CoV and SARS-CoV-2, the trimer bundle is much tighter and did not unravel during antibody binding. This is consistent with the distortion during cryo-electron microscopy and likely explains the lack of neutralization by the B6 antibody. Another study by Garrett et al., which located a number of linear binding epitopes in this region was unable to locate the cryptic epitope Sauer et al. describe, most likely because it is hidden by the stem helix bundle.
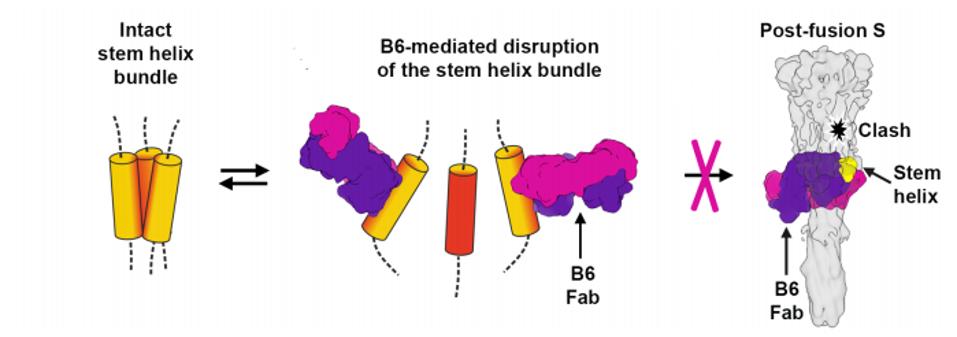
FIGURE 4: B6 unwrapping the stem helix bundle.
SAUER ET AL
What good is an antibody that does not neutralize SARS-CoV-2, as we are in the midst of a pandemic? We may have a way around this shortcoming. In a study by Koenig et al., a nanobody derived from camelid mammals was able to neutralize SARS-CoV-2 by binding tightly to the receptor-binding domain. The nanobody locked the receptor-binding domain in the open configuration and nullified its binding to ACE2 receptors. Why is this relevant? Perhaps when combined with this camelid nanobody, they will open the structure, exposing the cryptic epitope hidden by the tightly bound stem helix bundle in SARS-CoV and SARS-CoV-2. Then, B6 could be able to enter SARS viruses and neutralize them.
This combination therapy would, however, be dependent on the conservation of the targetted region: positions 1147 to 1161 (Figure 5). In the GISAID database, there are some mutations in these areas, but many are likely dead viruses as they include long strings of deletions or insertions. The most common mutations include H1159Y, which has been sequenced 329 times, S1147L, which has been sequenced 385 times, and D1153Y, which has been sequenced 689 times. While these are no insignificant numbers of sequencing, none of these mutations appear in major variants of interest or concern, which suggests that this region is significantly conserved, not only cryogenically, but also in over 1.5 million GISAID entries.
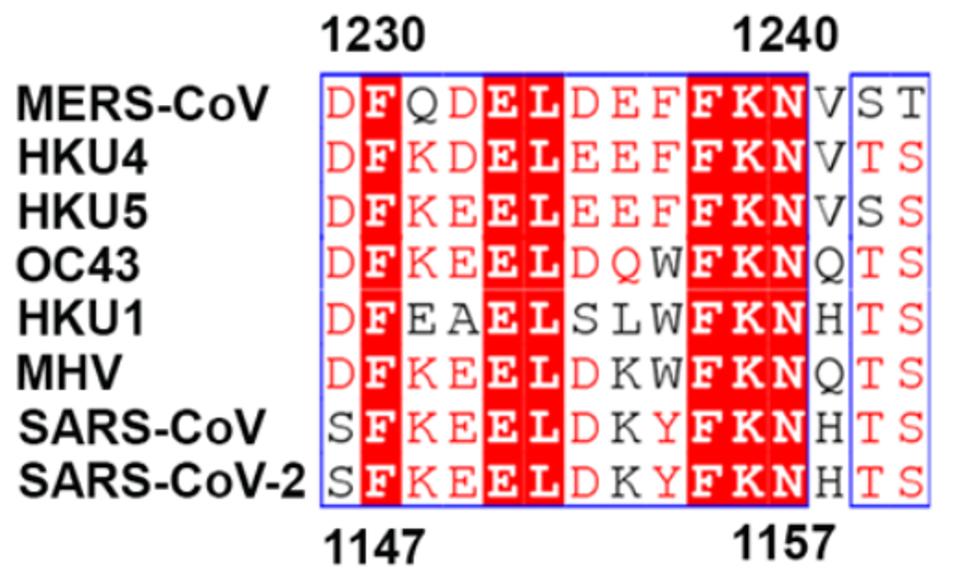
FIGURE 5: Conserved region among coronaviruses.
SAUER ET AL
This reminds me very much of the first effective vaccine against the feline leukemia retrovirus that I made in the early 1980s. The peptide in use was a membrane-spanning peptide that covered the carboxy terminus of the exterior glycoprotein, which deleted the membrane-spanning region, and included a small portion of the cytoplasmic domain. This vaccine provided then and still provides broad protection against leukemia in cats. That shows that peptide vaccines to conserved regions of spike proteins can be very important vaccines. Therefore, as the authors imply, peptides spanning this region may play a role in neutralizing many different variants. We echo their calls for increased research on this and other antibodies in the name of pandemic preparedness, as well as to find combination therapies that may control the SARS-CoV-2 virus today.
Read the full article on Forbes
Originally published on May 19, 2021

