Can We End the Pandemic?
(Posted on Friday, June 4, 2021)
The emergence of dangerous new coronavirus variants is threatening the progress that we have made with the help of COVID-19 vaccines. It is now clear that our pharmaceutical research efforts and public-health interventions will need to be redoubled – and without any sunset clauses.
At the start of the year, there was reason to hope that we were beginning to see the end of the COVID-19 pandemic. In the United States, the daily rate of new cases from the holiday surge began to drop precipitously, and the vaccine rollout accelerated soon thereafter. Although Europe lagged in the early months of 2020, there, too, the tide started to turn by April, as the pace of vaccination finally picked up.
But now that summer is almost upon us, the tide has turned once again, and not in our favor. New infections have been skyrocketing across South America, with countries like Argentina, Brazil, and Colombia approaching all-time highs of new infections in the past week. India crossed its own grim milestone last month with more than 400,000 new infections in a single day – a nearly unimaginable surge that overwhelmed hospitals and left families scrambling for any kind of care, oxygen, or medicines they could find. Even here in the US, states like Michigan and Oregon have struggled with recent spikes.
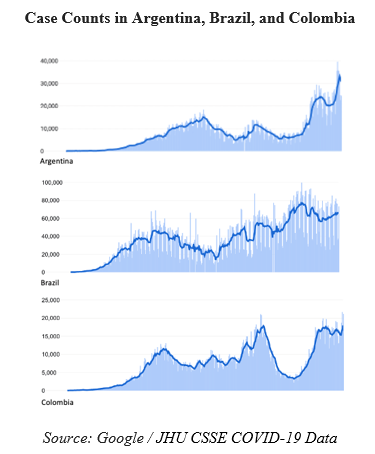
These resurgences should remind us that even with millions of vaccines being administered in some countries, the pandemic is far from over. We are certainly not back where we started, but nor can we ignore the challenges that await us.
MARKING THE VICTORIES
The good news is that the first generation of mRNA-based COVID-19 vaccines (from Pfizer-BioNTech and Moderna) are working even better than most had hoped. Not only do they protect against mild, moderate, and severe disease, but recent evidence suggests they also protect against infection. They have been proven effective in those 12 years and older and are already being tested in children as young as six. Even if the other vaccines don’t perform up to the same standard, having two vaccines capable of removing transmission from the equation will do wonders for pandemic control.
That said, we don’t yet know how long the protection from these vaccines will last, especially against new variants of SARS-CoV-2, the virus that causes COVID-19. The latest studies suggest that the strength and duration of vaccine-mediated immunity depends on the initial concentration of neutralizing antibodies following completion of immunization. The higher the concentration, the more potent and longer-lasting the protection – against both the original strain and new variants.
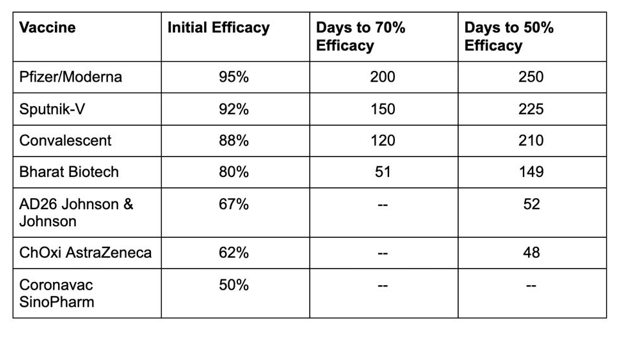
Again, though, not all COVID-19 vaccines are created equal. According to one preprint study that used existing data and statistical modeling to predict the performance of seven vaccines, the protection offered by the Moderna and Pfizer-BioNTech vaccines – currently the most powerful of the bunch, with 95% efficacy – could last as long as nine months, whereas vaccines with under 70% initial efficacy might lose their potency much faster. That doesn’t bode well for the widely distributed Johnson & Johnson and Oxford-AstraZeneca vaccines.
How do these predictions compare to what we know about natural immunity? One answer, based on a recent Singapore-based study, is that natural immunity is more variable than vaccine-furnished immunity. Researchers found that COVID-19 patients fall into five groups: those whose antibodies never reached detectable levels; those whose antibody levels were detectable within 20 days of infection, but dropped in fewer than 180 days; those who still tested antibody-positive 180 days after infection; those whose antibody levels showed little to no sign of decay for many months; and those who, against all odds, experienced a surge in antibody levels later in their recovery, as opposed to soon after infection. Most patients in the study ended up in the middle three groups.
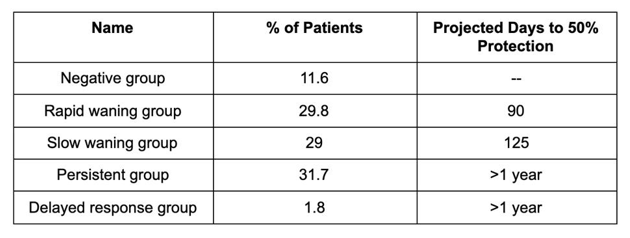
What we are looking at is a vast patchwork of immune responses. This variability means that there is no dependable front of recovered patients confronting SARS-CoV-2. Together with the uneven pace of vaccinations worldwide (not to mention the disproportionate allocation of the most efficacious vaccines), this could make herd immunity more difficult to achieve.
OTHER BREAKTHROUGHS
Beyond vaccines, there has also been progress in the development of prophylactic drugs that can prevent infection and disease from SARS-CoV-2. In recent clinical trials, monoclonal antibody treatments developed by Eli Lilly and Regeneron both performed well in nursing homes, reducing the risk of contracting symptomatic COVID-19 by 80% and 100%, respectively. Owing to drug resistance among new variants of the virus, the FDA has since halted the use of the Eli Lilly treatment, bamlanivimab, in isolation; but the drug may still be used in combination with others, and thus remains viable when deployed strategically.
The downside of these treatments is that they must be administered through intravenous infusions. But on April 12, Regeneron released the results of a clinical trial testing the drug by subcutaneous injection. In this case, the risk of symptomatic infections was reduced by 81%, on average.
It would of course be even better if prophylactics could be administered orally, like the pre-exposure prophylaxis for HIV. The two most promising candidates here are the antiviral molnupiravir and a protease inhibitor currently in development by Pfizer. According to a press release published by Merck, one of the companies behind molnupiravir, the drug performed well in a small case-control study of adults: none of those who received a dose tested positive for the virus, whereas 24% of the placebo group did./p>
Like molnupiravir, Pfizer’s protease inhibitor is intended to be administered orally and would be ideal for workplaces, schools, and congregate living environments, including long-term care facilities, prisons, and family homes. Integrating prophylactic drugs like these into our public-health strategies would help seal up some of the cracks in the current vaccination strategy. With several other promising antiviral drug candidates under development, the long-term hope is that some combination of oral prophylactics will reliably prevent infections in those who are exposed.=
COVID’S REVENGE
Set against this good news in drug development is the fact that new variants are constantly emerging. From what we know so far, almost all are more infectious, many have the ability to evade or mitigate the immune response (be it natural or vaccine-mediated), and some are more virulent (capable of causing disease in the host)
To understand SARS-CoV-2’s variants, it helps to look at the entire life cycle of the virus. Regardless of whether the virus moves through the air, by intimate contact, or via a fecal-oral or fecal-aerosol route, it must survive the journey so that it can attach to and enter a cell on a mucosal surface. Successful transmission thus depends on the amount of virus shed by the infected person, the stability of the virus particle, its affinity for the receptors at the mucosal surface, and the efficiency of entry.
Once inside the cell, the virus then needs to reproduce and spread to other cells. The extent of disease will be determined by the rate of virus replication and the efficiency of the immune response. In those who have recovered from a prior infection or been vaccinated, the virus’s fate depends on the strength of the immune response and its own ability to evade it.
Each new variant can affect any number of these variables, from virus load, stability, and attachment to cell entry, replication, and evasion of the immune response. Several variants – including the most widespread one first detected in the United Kingdom, B.1.1.7 – have a higher affinity for the SARS receptor (ACE2), are less sensitive to neutralizing antibodies, and reproduce more prolifically than the parent virus. B.1.1.7 infections can last up to 14 days before the virus is cleared from the body. As such, the variant has been shown to be around 60% more transmissible and more than twice as deadly as the original strain (though at least two studies cast doubt on the latter finding).
Most of the research on new variants has focused on the coronavirus’s spike protein. This makes sense, because the protection offered by vaccines comes largely from antibodies recognizing and responding to it. Nonetheless, we should remember that increases in transmission and disease result from a combination of the interactions between all of the viral genes and proteins within the host, not just the spike protein. Changes that affect any part of the infectious cycle can and will contribute to transmission and virulence – for better or worse.
SARS-CoV-2’s ability to evade the immune system’s response is an emerging area of study. Insofar as the initial immune response acts as an early-warning system, it is in the virus’s best interest to develop ways to slow down the system or slip through its grip. Unfortunately for us, SARS-CoV-2 has a trove of proteins it can devote to this task, as well as a special microcellular compartment that obscures its intracellular activities from the immune system, allowing it to produce double-stranded RNA unseen.
The virus can also disguise itself by mimicking the host cell’s own messenger RNA, thus evading a second trigger of the innate immune response. And SARS-CoV-2 has a set of “accessory proteins” capable of foiling specific early-warning signals that normally trigger a prompt immune response. Among these are proteins that inhibit interferon production and activity, which is the primary sign to the immune system that an invader has arrived.
Worse, mutations in SARS-CoV-2’s accessory genes may lead to more efficient viral replication and prolonged virus growth. The result would be an increase in the number of virus particles shed, leading to increased transmission and – not incidentally – more serious disease and deaths.
These are not theoretical conjectures. All the variants of concern spread rapidly in the population and carry mutations in many of these genes – including those that mask viral RNAs and that modulate the immune system to the virus’s advantage. The same variants also feature mutations that accelerate viral replication.
The effect of other mutations, especially those that modulate immune function, can only be evaluated in animals that closely mimic the human disease. To date, almost all of our knowledge of these variants is derived either from epidemiological studies or analyses of the virus in cell cultures. We would do well to study the contribution of each of the mutations in all of the viral genes to determine the overall transmissibility, potential to evade immune responses, and virulence of the variants.
When the new variants first began to emerge, many experts – including me – worried that they would eventually become dominant, disrupting public-health interventions around the world. Sadly, this fear has since been borne out. On April 7, 2021, the US Centers for Disease Control and Prevention reported that B.1.1.7 had become the most common SARS-CoV-2 strain in the US. And the same variant is tied to almost 75% of new cases in France and a doubling of case counts in Germany.
Moreover, over 50% of new infections in the US now trace back to a collection of new variants, some imported and at least three homegrown. In early March, a second-generation version of B.1.1.7 that carries an additional spike mutation – the feared E484K, nicknamed Eek! – was discovered in Oregon. And since then, a variant spreading rapidly in India, B.1.617, has reached the US, causing a growing percentage of infections in California and a sublineage, B.1.617.2, is spreading quickly in the UK.
New variants of concern have also been detected in Belgium, the Philippines, and Tanzania. Surprisingly, the Tanzania variant, which was first discovered in a traveler to Angola, derives from the SARS-CoV-2 A lineage, not the B lineage that prevails in much of the world.
ADAPTING TO THE ADAPTATIONS
Some have suggested that the coronavirus can have only so many tricks up its sleeve. But in light of how thoroughly the variants have blindsided us, this seems like a naive assumption. Looking across the topology of the SARS-CoV-2 genome, there appear to be endless possibilities for the generation of novel viable variants. Like the influenza viruses that never seem to run out of ways to evade the previous year’s vaccines, cold-causing coronaviruses evade our immune responses every year.
Moreover, humans aren’t SARS-CoV-2’s only host species, and the evidence suggests that some of the new variants have an even broader range of hosts than their predecessors. Among the latest animals to join the ranks, according to data from French researchers, are common breeds of laboratory mice – a finding disturbing in and of itself when we consider how densely these animals populate our cities. All told, scientists already have identified 80 species that are very likely to serve as welcoming hosts for SARS-CoV-2.
In other words, COVID-19 is on its way to becoming endemic not just to Homo sapiens but also to many other animals with whom we share an ecosystem. The greater the host range, the more opportunities the virus has to incubate in animal reservoirs without human interference – only to come back to haunt us in a deadlier, more infectious form. This phenomenon has already been documented on Danish mink farms, where the virus infected farmers, crossed over into minks, and then leaped back into human handlers.
Now that multiple variants have caught the world by surprise, we need to invest in a more systematized, standardized, and actionable approach to surveillance and detection. International cooperation will be crucial to making this a reality, as not all countries have the funds or infrastructure in place to do it alone.
First and foremost, this challenge demands a vast expansion of genome-sequencing efforts, so that we can detect and anticipate patterns in viral variation before they interfere with ongoing public-health interventions. This calls for more ongoing testing, an area where the US has faltered, despite the proliferation of technologies and evidence-based models that would make mass diagnostics feasible. Still, as of mid-April, the US government had committed $1.7 billion to improving variant surveillance through genomic epidemiology research initiatives and a National Bioinformatics Infrastructure for data collection. Such frameworks will increase responsiveness both to the current pandemic and to pathogens that lie in our future.
Equally promising, the UK is beginning to roll out a home-testing program that will allow everyone to test themselves at least twice per week for free. Britain also continues to lead the world in genomic-sequencing capabilities, thanks to the COVID-19 Genomics UK Consortium, a year-old group that has succeeded in looping its own data-collection processes into existing health systems (namely, testing programs in hospitals and community care centers).
BOOSTERS AND BEYOND
The first generation of COVID-19 vaccines was developed using essentially the same antigen. As such, the major difference between each vaccine is the strength of the antibody reaction it elicits – as demonstrated by studies linking the strength and length of vaccine-mediated immune protection to that of the antibody response. In terms of the current vaccines’ ability to protect against the new variants, however, a reduction in efficacy has been observed across the board.
The second generation of vaccines is already in development, with the focus primarily on the B.1.351 and P.1 variants, since these both have the immune-evasive E484K mutation. With the third generation, there will be an opportunity to create vaccines that are more broadly protective, raising higher, longer-lasting antibody titers. A platform in its early stages that has already shown promise in this regard is known as nanoparticle immunization technology, which combines fragments of different viruses into a single particle for loading into a shot.
Two research papers covering two approaches to developing a nanoparticle vaccine have been published to date. The first study uses a ferritin-based cage to aggregate bits of spike protein from different viruses, while the second study uses nanoparticles of the “mosaic” variety, consisting of 60 spike protein fragments that were identical in appearance and function, but were taken from up to eight different coronaviruses. Both studies found that in animal models – macaque monkeys in the first, and mice in the second – the vaccines performed remarkably well.
Recall that SARS-CoV-2 disarms our immune defenses through a highly coordinated attack that weaponizes the entire genome against us. As such, one drug – and target – isn’t enough. What we need is a combination of drugs that inhibits a broader spectrum of activity, and that can be taken orally.
But even if we have viable drugs on hand, we still need a strategy for combining and delivering them in conjunction with vaccines and other public-health interventions. The best use of orally ingested drugs, for instance, would be in congregate living environments like dormitories, nursing homes, and prisons, where they are easy to administer quickly and widely in the event of infection.
PERMANENT PREPARATION
Looking ahead, we must not repeat the mistake we made after the first two lethal coronavirus pandemics this century, SARS and MERS, when we allowed initial waves of research to recede. Pandemic preparedness and international cooperation must not be abandoned out of a false sense of safety.
In some countries – like New Zealand, Australia, China, and Singapore – public-health measures have been sufficient to control the pandemic. Their strategy is simple but rigorous, involving broad surveillance, rapid case identification, contact tracing, assisted isolation, and strict border control. But even this is not bulletproof. Singapore, for example, has just recently reinstituted a lockdown, shutting down schools, restaurants, and other public gathering places after a spike of new infections tied to the B.1.167 variant.
For countries like Singapore, vaccination is a critical backstop for COVID-19 containment. In countries that have enforced various combinations of these public-health measures with much less success, hopes now hinge mainly on mass vaccination and drug development. We need to increase our capacity for vaccine production and invest in more expansive, long-term solutions like mosaic vaccines. We also need to broaden existing vaccine-distribution channels so that no country is left behind. The virus still poses a grave threat in many countries, which means it still poses a grave threat to us all.
Read full article on Project Syndicate
Originally published in Project Syndicate (June 4, 2021)

