What We Need To Know About The Future Of Variants
(Posted on Thursday, July 29, 2021)

NEW YORK CITY – JULY 27: A person wears a mask while walking in Grand Central Terminal on July 27, 2021 in New York City. Due to the rapidly spreading Delta variant, the Centers for Disease Control and Prevention (CDC) has recommended that fully
GETTY IMAGES
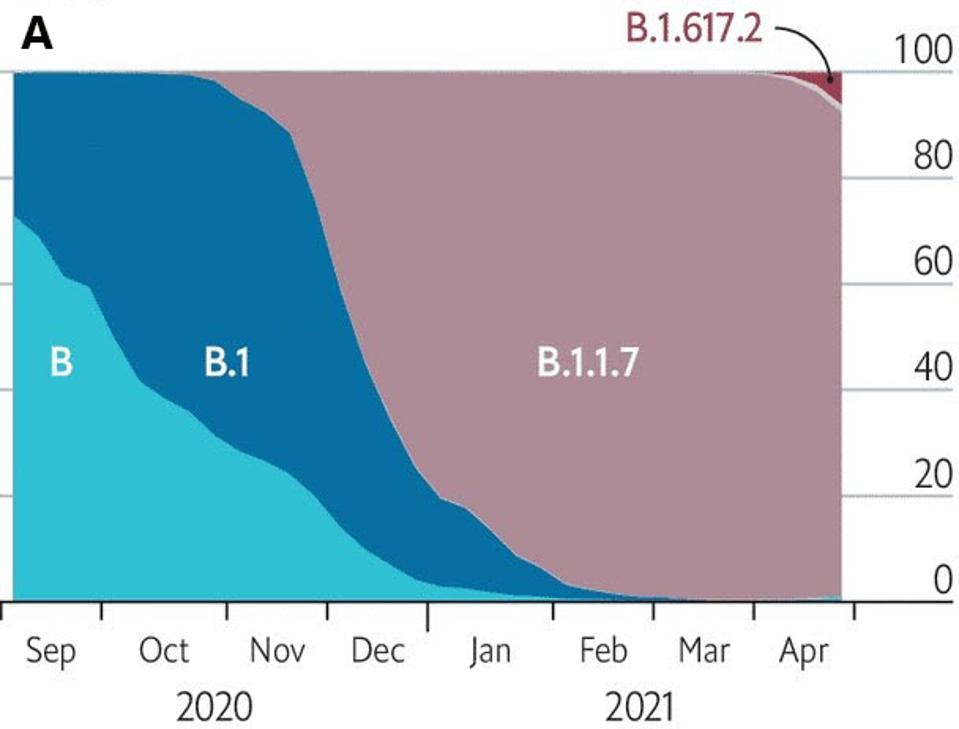
For the past year, variants of SARS-CoV-2 have upset our best-laid plans for recovery. As I write, the global spread of the Delta variant has darkened prospects for an early end to the pandemic. Delta outstaged the Alpha variant in recent months, overtaking it as the most prevalent strain in SARS-CoV-2 infections (Figure 1). Virologists disagree on the following question: is Delta as destructive as they come, or is an even more potent variant on the horizon?
THE ECONOMIST
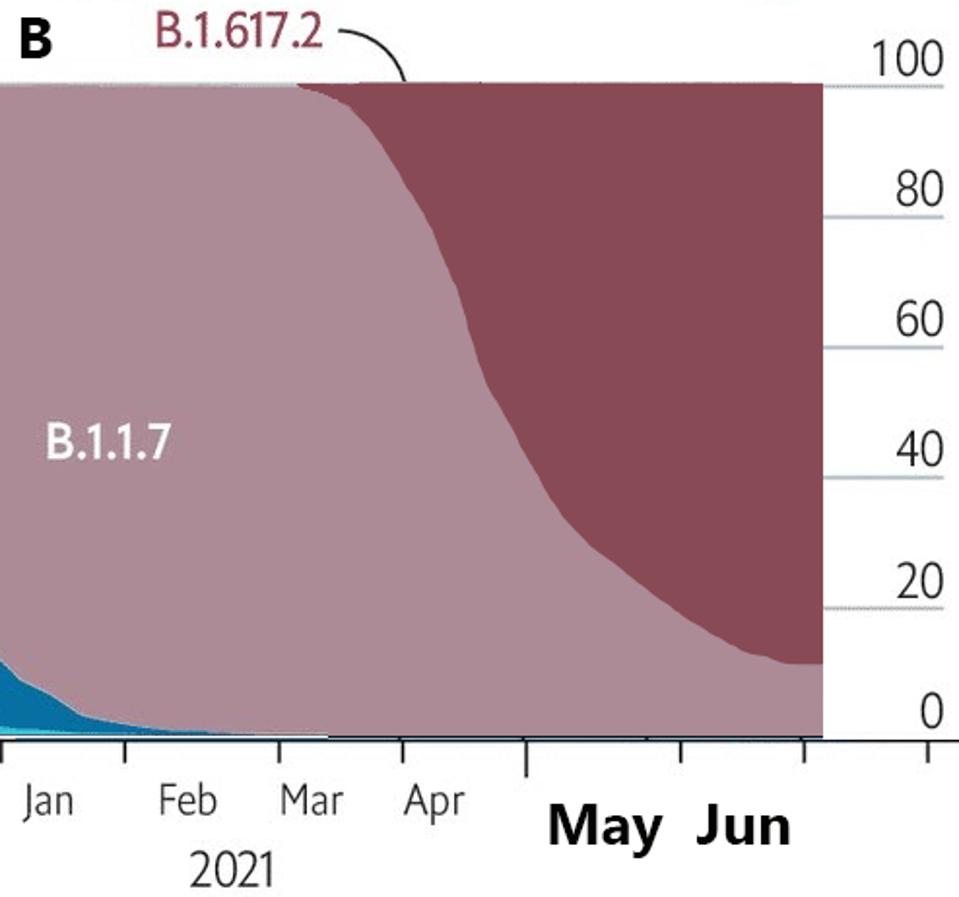
FIGURE 1: A: Rise of B.1.1.7 in England from September 2020 to April 2021. B: Rise of B.1.617 in England from January 2021 to June 2021.
THE ECONOMIST
Some argue that SARS-CoV-2 has a limited repertoire. Others, including me, believe that the virus has a capacity for change that exceeds anything we have seen to date. The optimists focus on recurring independent mutations in the Spike protein as evidence of a limited capacity for change. If that is true, a broadly protective vaccine may be just around the corner. Those less optimistic look to our failure to create a pan-protective influenza vaccine, one requiring annual re-vaccination to keep pace with influenza variants.
In time, we will have an answer. Until then, we can only seek to understand SARS-CoV-2 in greater detail, both by an intensive study of the virus itself and by analogy, with what we know from the study of other viruses. I am distressed to report that even now, with all the marvelous tools at our disposal, we know too little to answer the question, but what we do know I find disturbing.
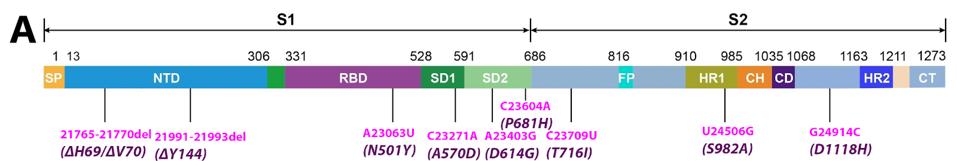
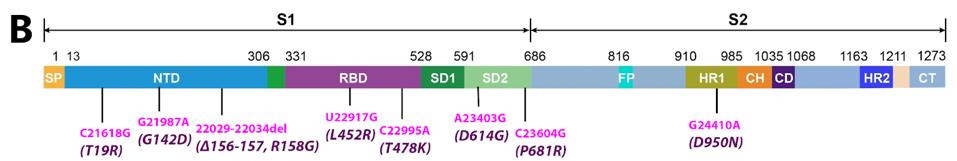
A cursory examination of the SARS-CoV-2 variants reveals that there are as many mutations (nucleotide changes) in non-Spike protein areas of the genome as there are in the Spike protein itself (Figure 2). Many of these mutations likely affect the structure and function of viral genes in addition to the Spike protein. Almost all discussions of variants either ignore or dismiss the potential relevance of these mutations. Given what we know about viral replication and pathological subtleties, this seems to be a serious oversight. The subtle difference between the virulence and vaccine genomes of polio makes the point. The critical difference between a pandemic strain of poliovirus and the live attenuated vaccine, the Sabin vaccine, is a single nucleotide change that does not change a single amino acid!
ACCESS HEALTH INTERNATIONAL
ACCESS HEALTH INTERNATIONAL
ACCESS HEALTH INTERNATIONAL
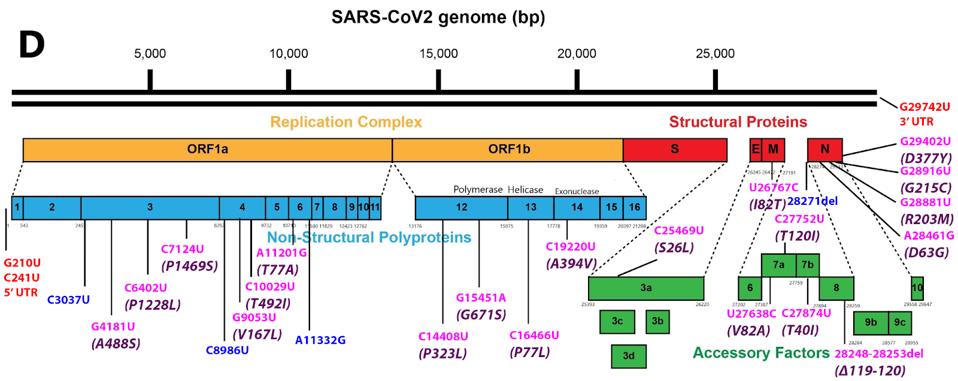
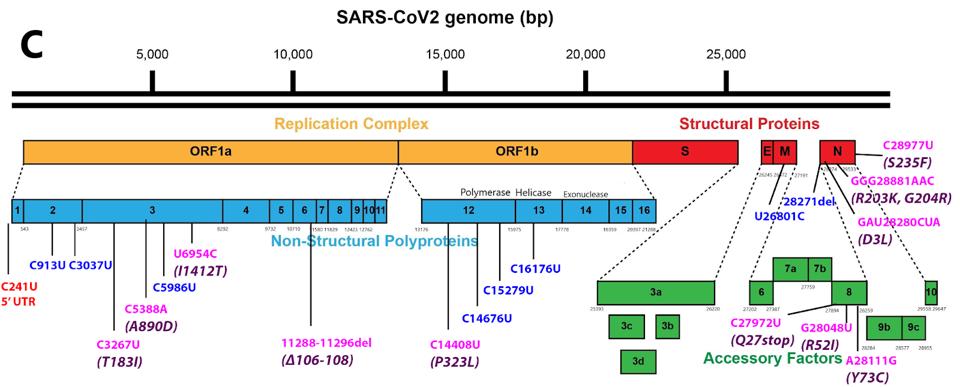
FIGURE 2: A: Nucleotide changes in the Alpha variant Spike protein. Pink denotes nonsynonymous mutations. Purple denotes amino acid mutations caused by nonsynonymous nucleotide mutations. B: Nucleotide changes in the Delta variant Spike protein.
ACCESS HEALTH INTERNATIONAL
A recent paper by Thorne et al. reiterates the warning of impending danger. The researchers found that a mutation in the Alpha variant that changes a single amino acid in the N protein gene results in an 80 fold overexpression of the overlapping gene for the Orf9b messenger RNA. The authors attribute overproduction not to a significant change in the function of the N protein, but rather to the underlying change in the nucleotide sequence that dramatically increases the relative abundance of the Orf9b messenger RNA. They also speculate that the overproduction of Orf9b protein may increase the transmission and lethality of the Alpha variant. Why? Because the Orf9b protein contributes to suppression of the innate immunity allowing the virus more time to replicate before immune recognition impedes its progress. It is worth noting that another suppressor of innate immunity, Orf6, is also over-expressed by the Alpha variant. Why remains a mystery.
Understanding the contributions of mutations to the epidemiology and pathogenesis of the Alpha variant is like looking through glass in the dark. We lack essential information about SARS-CoV-2 to guide us. Similarly, the detailed information regarding the structure and function of HIV that guides our ability to develop new drugs to prevent and treat AIDS infections is lacking. Underinvestment in coronavirus research hampers our ability to predict where and how the next variant may emerge. One remedy is to dramatically increase the budget for fundamental research on coronaviruses, a strategy I successfully championed for HIV in 1985. Another is to search for clues in other better-studied viruses. Here are three examples from the study of three different viruses that illustrate how subtle genomic mutations can have unexpected effects on viral replication and pathogenesis.
Example 1. Most influenza vaccines today are dead-virus preparations made from large batches. Vaccinologists discovered that fragments of the virus genome completed with the whole virus dramatically decreasing viral yield. The race was on to understand what favored replication of these fragments over that the whole genome. Scientists were surprised when they tracked the culprit to several single amino acid changes in the viral NS2 gene, which until then believed to function only to shepherd viral RNA out of the nucleus and not play a direct role in genome replication.
Example 2. In an attempt to adapt specific strains of hepatitis C to growth in cell culture for research purposes, Chan et al. isolated a strain that replicated to a 1000-fold higher titer in cell culture than its parent. Further analysis revealed that subtle amino acid changes in six viral proteins, including structural and nonstructural proteins, were responsible. The major contribution was from a nonstructural protein that stabilizes and increases the half-life of the free virus particle by three to four-fold. However, the sum of the individual contributions to increased infectivity was less than that of the ensemble.
Example 3. The third example is from another coronavirus, one that causes hepatitis in mice. In seeking to understand the signals required for the production of new copies of the viral genome, researchers introduced a mutation that almost eliminated the ability of the virus to grow. Eventually, strains emerged that grew rapidly despite the deleterious mutation. They then sought to discover how the virus overcame its disadvantage. The answer came as a complete surprise. Mutations in the genes encoding viral proteins, NSP8 and NSP9, were responsible. These two genes are located far from the site of the original mutation. The experiment revealed previously unsuspected roles of these two proteins in virus replication.
The lesson for the study of SARS-CoV-2 variants is clear. Research should broaden the focus from studies of the Spike protein alone to include studies of every other protein, both singularly and multiple combinations, both concerning virus replication in tissue culture and organoids, as well as the ability to evade immune recognition.
I raise these three examples to illustrate the complexity and subtleties involved in understanding increases in virus transmission and virulence. We should not underestimate our viral foe. Until we know much, much more than we do today of fundamental aspects of coronavirus replication, pathogenesis, and variation, our watchword must be vigilance, not complacency. A virus far more transmissible and dangerous than Delta may be on its way.
Read the full article on Forbes, (originally published on July 29, 2021).

