Is An Artificial Pancreas On The Way?
(Posted on Tuesday, September 28, 2021)
This story is part 1 of an occasional series on the current progression in Regenerative Medicine. In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.

A recent study shows real promise towards a bioartificial pancreas.
PIXABAY
A recent study shows real promise towards needle-free management of type 1 diabetes. In July, a research team at Brigham and Women’s Hospital together with scientists from Harvard University and the University of Massachusetts described the design of an artificial pancreas for children and adults with type 1 diabetes. The device successfully passed many of the tests required for first trials in patients.
As of 2014, over 38 million people worldwide have type 1 diabetes and the prevalence of this disease seems to be on the rise. Once patients receive a diagnosis, they must follow highly regimented diets, closely monitor their blood sugar levels, and take daily insulin injections. Although these methods have improved the lives of those with type 1 diabetes, there is still a need for a more natural solution for sensing and producing insulin.
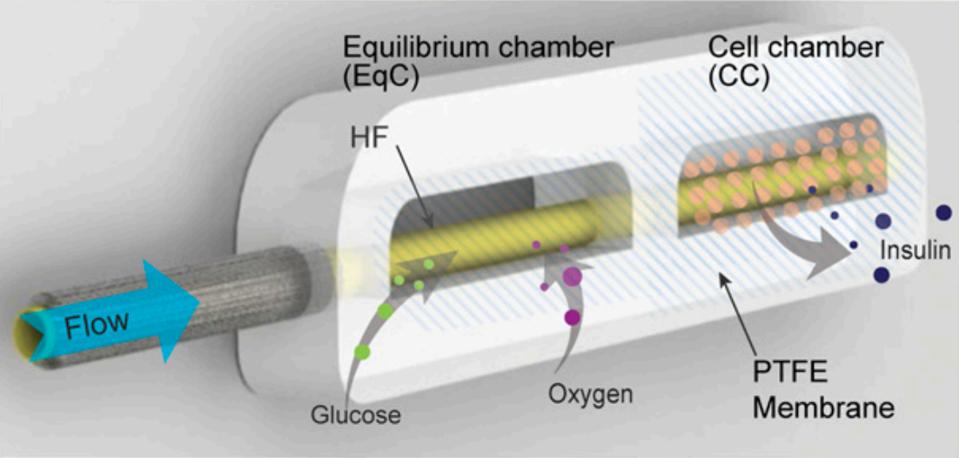
So, how can this device help? The artificial pancreas is a small, implantable device that automatically produces insulin according to the body’s needs. The device consists of a chamber that houses live insulin-producing islet cells. The walls of the chamber protect the islet cells from the body’s immune system. The novel feature of the device is a built-in pump that circulates nutrients and oxygen to the chamber cells so they can thrive and continue to produce insulin.
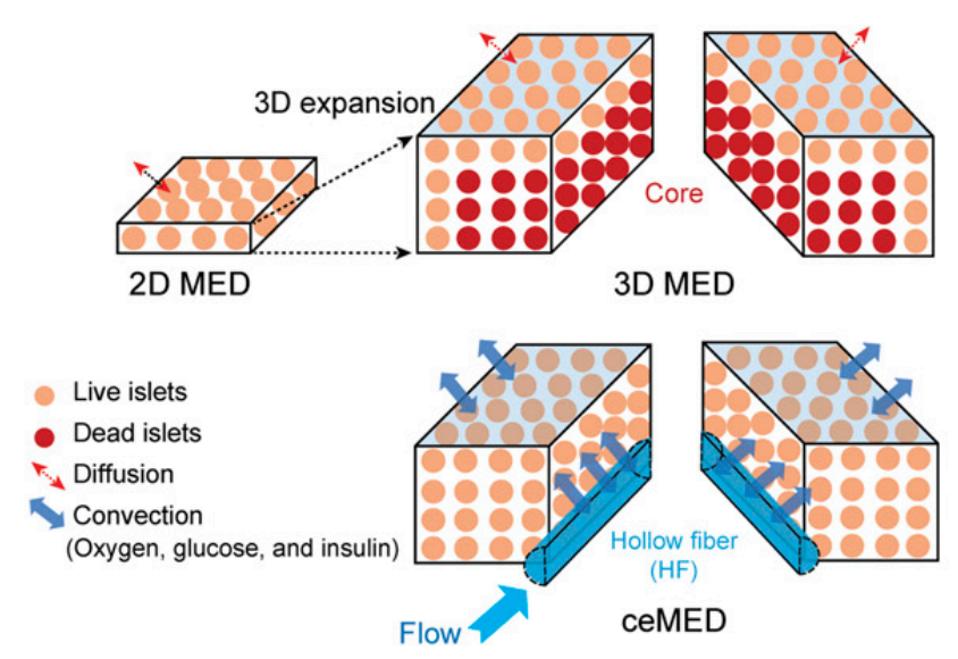
This new device is designed to address the primary issue of ensuring that cells receive necessary oxygen and nutrients. Previous encapsulation devices relied on diffusion alone to transport nutrients to cells. However, the rate of diffusion proved insufficient to provide cells with enough necessary oxygen, leaving most of the cells dead. Researchers believe that a pump that actively circulates both nutrients and oxygen through the device will address this issue.
The research team tested their theory by first designing a prototype. The prototype consists of two chambers connected by a tube. The first chamber collects a supply of nutrients including oxygen and glucose. This chamber has a membrane with larger pores to allow for more nutrients to pass through. Directly following this chamber is a second compartment that contains the beta cells. The second compartment is surrounded by two membranes that have smaller pores. The smaller pores filter out cells and antibodies that could kill the sensitive beta cells.
Labeled image of device
PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES, K. YANG, E.D. O’CEARBHAILL, ET AL.
A tiny pump manages the flow of liquid to the chambers. In the first chamber, nutrients collected diffuse into the tube and are pushed along to the second compartment. The flow of nutrients causes high pressure and high nutrient concentration within the tube. This contrasts with the low pressure, low concentration environment of the second chamber. Because of the difference in pressure and concentration, necessary nutrients and oxygen reach the cells.
Convection vs. Diffusion Design
PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES, K. YANG, E.D. O’CEARBHAILL, ET AL.
The first test was to determine whether cells could survive in the device. The team began their experimentation with insulin-producing MIN6 cells. The test compared active flow cells to those with passive diffusion. The active flow cells survived at a much higher rate and produced insulin more readily in the presence of glucose than cells with passive diffusion.
Having overcome the first hurdle, the team moved on to a cell that was more similar to the cells that may actually be used in humans—stem-cell-derived beta cells (SC-βC’s). These cells are insulin-producing cells derived by Professor Douglas Merton from Harvard University. The SC-βC’s with flow survived at higher rates in the prototype and continued to produce insulin, while cells without flow did not.
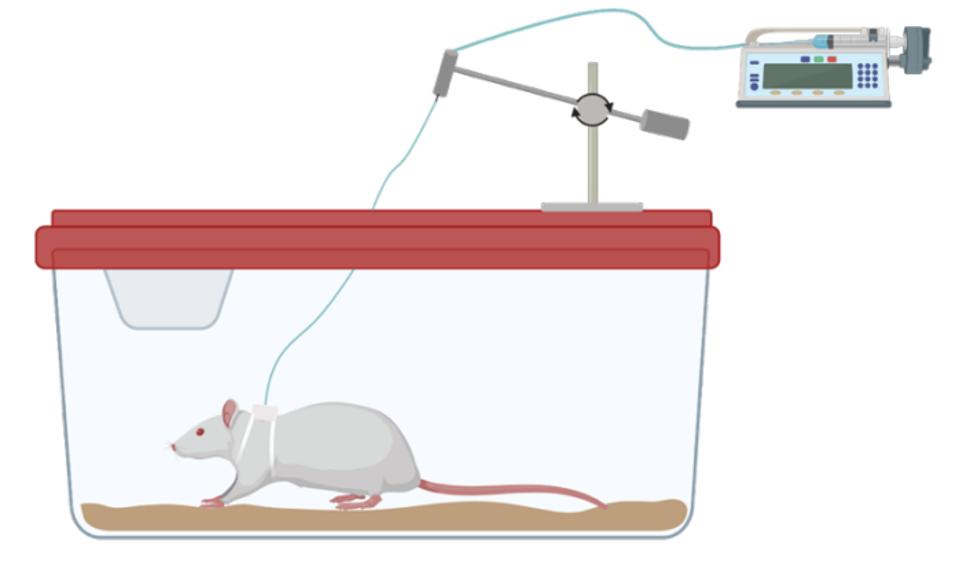
After additional experiments were conducted to determine the maximum number of SC-βC’s that could be housed by the device, the team was ready to begin testing the devices in live rats. Scientists surgically implanted the device under the rat’s skin. They modified the device with a tube that ran from the rat to an external pump. Researchers used the pump to accurately infuse certain glucose levels into the animals.
Rat experimentation set-up.
PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES, K. YANG, E.D. O’CEARBHAILL, ET AL.
Trials of the active flow device in rats with diabetes were highly successful at maintaining the viability and insulin secretion activity of SC-βC’s compared to rats with diffusion devices and rats with no devices. Cells could also accurately respond to large glucose fluctuations in the live animal.
Not only this, but the larger pores in the first chamber’s membrane promoted the growth of new blood vessels. This means that the device has the potential to be implanted in larger, less vascularized areas which would require less invasive surgeries. If blood vessels can successfully grow around the device, then cells would also have easier access to nutrients.
Further research must be conducted to determine the optimal dimensions of the device in live animals. More experimentation must also occur before the device can be available for humans. However, this publication signals an optimistic future for the decade’s old dream of allowing people with diabetes to control blood sugar levels without the need for insulin supplements.
Originally published on September 28, 2021.

