Covid-19 Variation: Past, Present, And Future
(Posted on Thursday, October 14, 2021)
This is the first in a three-part series examining the past, present, and future of the pandemic and viral variants.

Proteus
GREEK ART
For the last year and a half, we’ve been consumed by successive waves of Covid-19 both in the United States and around the world. We have witnessed the rise of variants that are both more transmissible and more dangerous. Now as the fourth wave in the United States, driven by the Delta variant may be on the wane, it is time to reflect on our past and contemplate the future. In this three-part series on viral variants, we look at what has happened, what is happening, and what the future might hold.
By late January and early February 2020, the Chinese government sounded the alarm: there is a new infectious coronavirus that is highly transmissible and more lethal than Influenza. There were private communications at the highest level between the US and the Chinese government that the coronavirus was likely airborne. In reaction, the Chinese government took swift and rigorous action to contain the virus through stringent public health measures, including identification of those exposed and infected, as well as the implementation of aggressive quarantine strategies not only of individuals but in some cases entire cities and provinces. That prompt action contained the coronavirus within China and continues to contain it to this day.
Unfortunately, by that time, the virus had begun to spread globally, including Europe, North America, and Southeast Asia. Other nations did not institute standard public health measures as promptly or thoroughly as the Chinese. Consequently, most countries have experienced successive waves of SARS-CoV-2 infection. In retrospect, we now know that the Wuhan strain, while highly infectious and dangerous in the sense that 1.5 to 2 of 100 people died following infection, was the first in a succession of much more transmissible viruses.
In January 2020, a new strain of the virus became evident, most commonly identified by a mutation in the Spike protein, called D614G. This single amino acid change has two effects on increasing transmissibility of the virus. First, it stabilizes theS1/S2 complex after cleavage and secondly, it favors an open, rather than closed, configuration for the receptor-binding domain—the configuration required for infection.
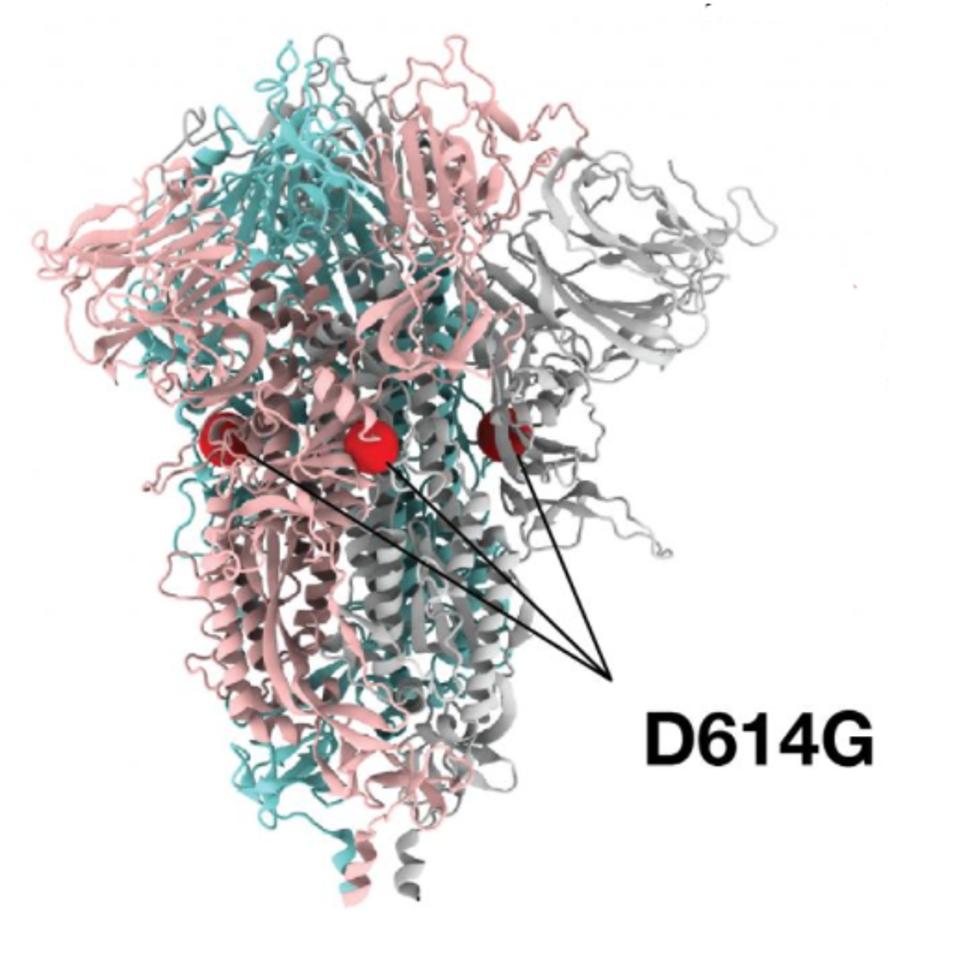
FIGURE 1: D614G mutation noted on the cryo-electron structure of the SARS-CoV-2 Spike protein.
LOS ALAMOS NATIONAL LABORATORY
Although D614G is featured as the predominant discussion of this early variant, we note that in addition, there are actually two other mutations that occurred, which today are inseparable from their D614G counterpart. These include an amino acid change observed in the NSP12 polymerase, P323L, and a single nucleotide change occurred in the five prime untranslated region, C241U. For this reason, we refer to this variant as the Triad variant. Intensive study of this Triad variant found that it was roughly twice as infectious as the original strain. In a matter of weeks, this modified virus displaced the Wuhan virus almost entirely across the world. The takeover of the Triad variant is virtually complete by May 2020 and is the primary driver of the wave of infections that hit in Summer 2020. Many states in the United States and countries around the world failed to implement public health measures to halt viral spread and furthermore, those measures they did install were promptly removed state by state throughout 2020.
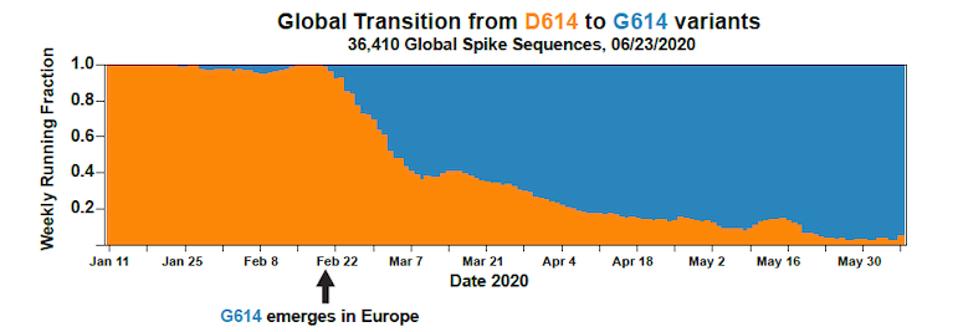
FIGURE 2: Transition from Wuhan virus to D614G mutation in SARS-CoV-2 infections in Europe.
LOS ALAMOS NATIONAL LABORATORY
At this point, many began to predict that the virus was stable and would not mutate further, based on expectations that the virus’s error-correcting machinery would eliminate further variance. Unfortunately, that expectation has turned out to be incorrect. In December 2020 and into January 2021, a number of new variants began to predominate. At first, these variants were largely regional, but all were derived from the Triad blueprint. Among these were the Alpha variant first identified in England, Beta first identified in South Africa, Gamma in Brazil, Lambda in Peru, Mu in Colombia, among others.
By Spring 2021, Alpha was the predominant strain around the world, barring South America in large part due to its aggressively transmissible nature. Beta became dominant in Southern Africa, but failed to gain global traction, despite a strong immune-evasion property. In South America, Gamma became dominant with a combination of transmissibility and immune-resistance, but was largely contained to the continent. In the United States, there were several different viruses that were circulating and formerly were variants of interest, such as Iota and Epsilon, but neither of them became totally dominant. Again, all of these Greek-alphabet of variants contain the first three mutations descended from the Triad variant, but each one had its own set of unique mutations.
ACCESS HEALTH INTERNATIONAL
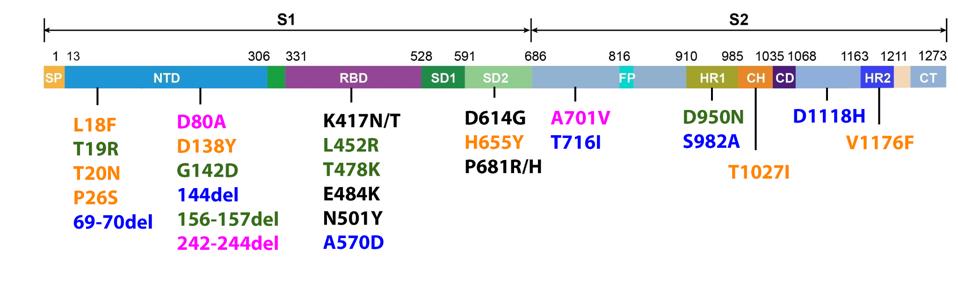
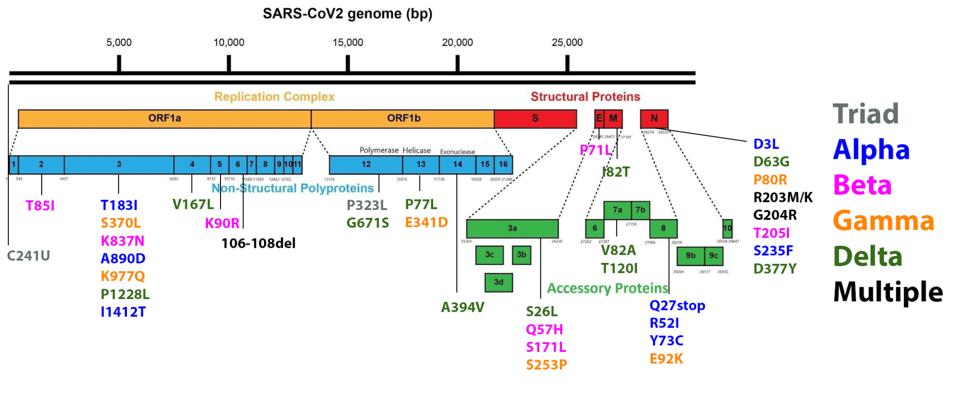
FIGURE 3: Mutations commonly observed in the full genome and Spike protein of SARS-CoV-2 in major variants. Triad mutations are noted in gray; Alpha in blue; Beta in pink; Gamma in orange; Delta in green; mutations found in multiple variants are
ACCESS HEALTH INTERNATIONAL
As we have noted repeatedly in our ongoing analysis of viral variants, it is important to monitor mutations outside the Spike protein with as much vigilance as those within the Spike protein. Any mutation which allows the virus to transmit more readily, must be carefully monitored, regardless of its viral location. For that reason we show figure three, which is the range of mutations that occur both within and outside the Spike protein in major variants. Please note that every major variant has significant mutations in the Orf1ab replicative complex, the non-spike structural proteins E, M, and N, and the regulatory protein. Any of these mutations has the potential to increase transmissibility and immune evasion. Some of these mutations modify highly antigenic proteins, such as Orf3a, which could affect convalescent or monoclonal antibody binding. These are just some of the many potential impacts non-spike mutations could confer, and thus they should be monitored intently.
Most recently, as we will describe in part three, the Delta variant, first identified in India, has other variants to become the world’s most dominant strain, even displacing Gamma as the dominant strain in Brazil, due in part to its elevated transmissibility over other variants.
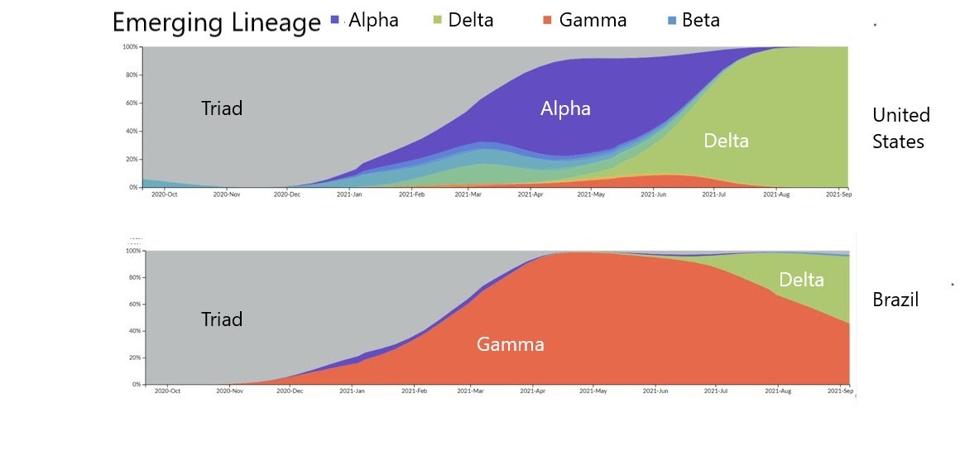
FIGURE 4: Transition between emerging lineages from the Triad variant to the emergence of the Delta variant in the United States and Brazil.
STEBBING
The figure above illustrates that SARS-CoV-2 is less so an isolated crisis, but an evolving offensive we must continue to counteract. Akin to how Influenza reemerges in different forms year on year, we can expect a similar pattern from SARS-CoV-2. What drives the evolution of variants? It is clear that in natural settings, each successive variant has transmission advantages over its predecessor. The Triad is more transmissible than the Wuhan strain. Each of Alpha, Beta, Gamma, and so on is more transmissible than the Triad. Alpha was more transmissible than other variants and displaced them, and now Delta has become the most transmissible, displacing nearly every other strain around the world.
These viral advantages are derived from mutations begin as one of the many billions of differences found in the swarm of SARS-CoV-2 viral particles. Over time, mutations that confer enhanced properties to the virus appear more and more until they are the nucleotide in the genome, akin to natural selection in humans and other fauna. Transmission advantages are among those selected to become dominant in the virus.
There may also be a selection for immune evasion. A number of changes, particularly in the Spike protein, allow the virus to escape monoclonal antibodies. These include, but are limited to N501Y in Alpha, Beta, and Gamma, E484K in Beta and Gamma, K417N/T in Beta and Gamma, and L452R in Delta. These are among the most well-researched antibody-resistance mutations, but others likely confer similar effects.
In addition, some of these Variants are less sensitive to neutralizing antibodies from convalescent sera, particularly Beta, which is also less sensitive to vaccines. All other variants seem to be relatively equally sensitive to vaccines. Therefore, with the possible exception of Beta, it may not be escape from neutralization that gives these viruses that advantage. Rather, there seem to be other factors, including replication for a longer period and replication to a higher concentration.
What gives a replication advantage to Alpha is not clear because they seem to be reasonably well neutralized by both convalescent sera and vaccination. There are subtle changes in the envelope glycoprotein that give it a better transmission advantage, but it is also possible that other mutations in nonspike proteins lead to other effects that should be investigated further.
The engine driving the emergence of variants is random mutation. It is estimated that there are about three nucleic acid substitutions per genome per replication cycle. If there are between 10 and 100 billion virus particles in any given infected person, that means there are 30 to 300 billion mutations in a given replication cycle. Many of those will either be dead or will have a replication disadvantage, but some will be stronger than the virus that came before. Emily Anthes of the New York Times describes this phenomenon as winning the genetic lottery, as very rarely does a new advantageous mutation emerge, but when it does, it does so overwhelmingly. Advantage mutations will be selected, further replicated, and new variants are formed, the strongest of which can infect the entire planet.
In the next entry, we will examine the evolution of the Gamma variant over the past several months in South America. In the final entry, we will examine the ongoing evolution of the Delta variant and its implications for the future of the pandemic.

