Omicron Evades Most But Fortunately Not All Monoclonal Antibodies
(Posted on Thursday, December 23, 2021)
This scanning electron microscope image shows SARS-CoV-2 -also known as 2019-nCoV, the virus that causes COVID-19-isolated from a patient in the U.S., emerging from the surface of cells cultured in the lab. (Photo by: IMAGE POINT FR)
UNIVERSAL IMAGES GROUP VIA GETTY IMAGES
The Omicron variant of SARS-CoV-2 has rapidly ricocheted around the world. Reported cases are at their highest since May 2021. There is a growing need for effective monoclonal antibody treatments to prevent and treat Omicron infections. Unfortunately, most approved anti-SARS-CoV-2 antibodies have a diminished potency or are ineffective against Omicron. The Omicron mutations which render the virus resistant to antibodies induced either by natural infection or vaccine also render them resistant to most monoclonal antibody treatments. Most does not mean all. At present, there are at least five well-characterized antibodies that retain potency against most variants of SARS-CoV-2 including Omicron.
S2K146
S2K146 is a monoclonal antibody developed jointly by Vir and GSK. Isolated from the memory B cells of a symptomatic Covid-19 patient, S2K146 bind the SARS-CoV-2 S protein, as well those of SARS-CoV-1, bat coronavirus WIV-1. Additionally, the antibody-bound and potently neutralized a number of variants of concern or interest, including Alpha, Beta, Gamma, Delta, Epsilon, and Lambda. Structurally, the antibody locks two of the trimer’s three receptor-binding domains in the open configuration while the remaining binding domains remain closed.
As outlined by Park et al., the S2K146 antibody footprint mimics that of the ACE2 binding sites. As many as 21 of the amino acids of the receptor binding site of the virus’s spike protein also bind the S2K146 antibody (Figure 1). These amino acids include heavily mutated sites in variants of concern, including N501, E484, and K417. Mutations of these mutations normally reduce antibody binding. The research speculates that neutralization is preserved as the interaction between the antibody and the receptor occurs over so many contacts. To me, it makes sense that an antibody has a structured surface that closely mimics that of the ACE2 neutralizers most variants. However much it may mutate, the spike protein must bind to ACE2 itself to initiate infection. I expect to see many more such ACE2 antibodies in the future, some with binding affinities that exceed that of S2K146.
CV3-1
The CV3-1 monoclonal antibody also neutralizes several variants of concern and may also retain activity against Omicron. The antibody binds to a structure designated the 485-GFN-487 loop in the receptor binding domain. The three amino acids are well conserved in Omicron and most other variants. (Figure 2, left)
The antibody recognizes the receptor binding domain in the open configuration (figure 2. right). Evidently binding of the antibody triggers a conformational change that mimics that of binding to the natural receptor. Upon binding, the S1 protein dissociates from the membrane-bound S2, laying bare the structures on the S2 protein that drives viral to cell membrane fusion required for virus entry. According to the researchers binding, by CV3-1 triggers premature release of the S1 protein from the virus surface, rending it incapable of infection. In other words, the antibody imitates ACE2 binding and springs a trap before the virus enters the cell. Rather than blocking binding as does S2K146, CV3-1 inactivates the virus prematurely. Wenwei et al. report that this loop structure is critical for S1 activation.
CV3-25
A third antibody CV3-25 binds to the S2 protein of the Spike. This region is highly conserved. among betacoronaviruses. Specifically, CV3-25 bindings the stem helix near the membrane attachment site (Figure 3).
Wenwei et al. find that the antibody binds to one or two units of the S protein trimer. The main site of interaction between CV3-25 and S2 lies between residues 1153 to 1165. More detailed analysis, including binding to an isolated peptide corresponding to this region, reveals that the antibody recognizes a linear epitope. A three-dimensional conformation is not required. The ability of the antibody to bind and neutralize the virus via a linear epitope raises the exciting possibility that it may be possible to develop a vaccine using the linear peptide that directs the immune response to this conserved neutralizing site, a possibility suggested by the authors.
Both CV3-1 and CV3-25 efficiently neutralize the Wuhan wildtype virus, as well as variants of concern Alpha, Beta, Gamma, Delta.
We have previously discussed other monoclonal antibodies for SARS-CoV-2 that could be used in conjunction with those described here to create a potently effective treatment. For example, a SARS-CoV-2 camelid nanobody binds across the receptor-binding domain in such a way that the trimer’s receptor-binding domains remain in the closed configuration.
Sotrovimab
Sotrovimab antibody was developed by Vir and is marketed by VIR/GSK. I have previously described this antibody here. The potency of Sotrovimab is only moderately diminished by Omicron as compared to its activity against other variant of interest. Sotrovimab is approved for emergency use in the United States and elsewhere. The epitopes to which Sotrovimab are moderately conserved in existing variants of concern. Whether Sotrovimab will retain activity against variants to come is uncertain.
Evusheld
Evusheld, developed by Astra-Zeneca, is a combination of two antibodies tixagevimab and cilgavimab, both of which were isolated from Covid-19 patients. The two antibodies bind to the receptor binding domain of the S1 protein albeit at the different and complementary sites (Figure 5). The antibodies were engineered to enhance their half-life. Evusheld is designed to be administered as an intramuscular injection, as opposed to an intravenous infusion.
Early data indicated that the combination antibody effectively neutralized variants of concern including the Delta variant. Recent studies show that Evusheld is substantially less effective against Omicron as compared to earlier variants. Nonetheless, Omicron retains what appears to be sufficient activity to provide at least some protection against Omicron.
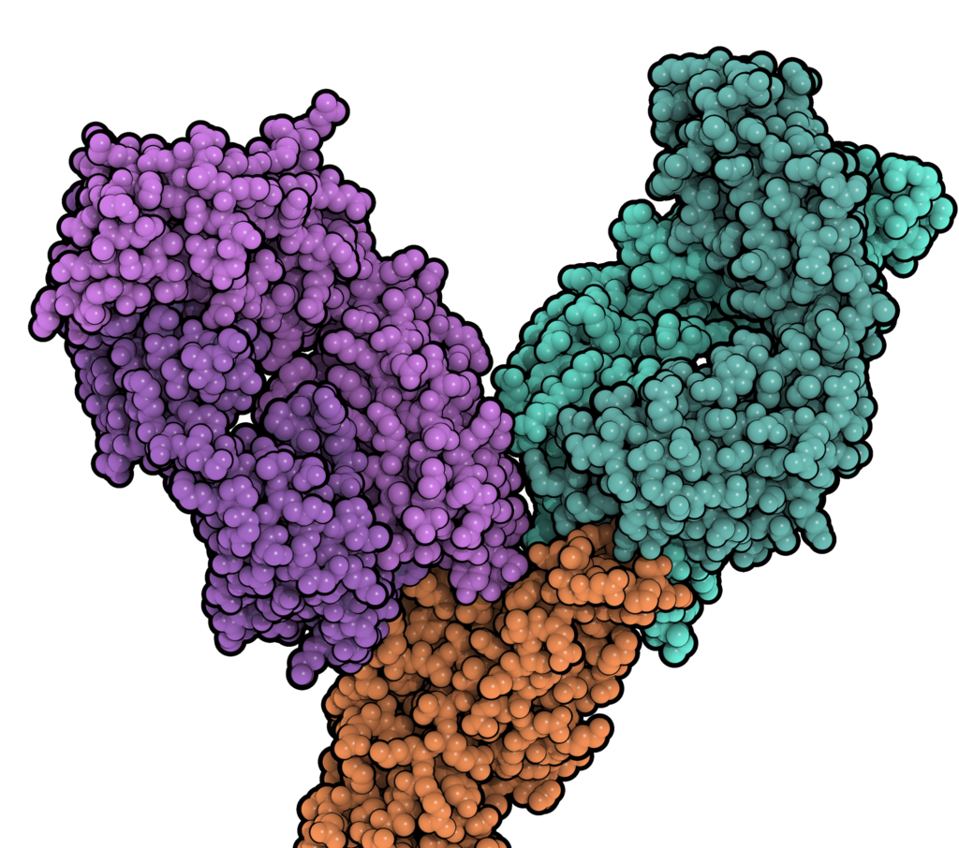
FIGURE 4: Tixagevimab (teal, right) and cilgavimab (purple, left) binding the spike protein RBD.
ACCESS HEALTH INTERNATIONAL
Conclusions
As potentially dangerous as Omicron may be— more transmissible than delta and resistant to most vaccines and monoclonal antibodies— at least two approved monoclonal antibody treatments remain in our arsenal; Sotrovimab, and Evusheld. More are in active development. The recent emergency use authorization of Paxlovid, a small-molecule antiviral protease inhibitor for the treatment of the infected vulnerable, is what we hope will be the first of many new drugs that in combination with broadly neutralizing antibodies may effectively treat and eventually prevent SARS-CoV-2 infections.
Read the full article on Forbes
Originally published on December 23, 2021

