New Potential Covid Virus Variants Of Concern
(Posted on Friday, January 7, 2022)
Regional Map of France
GEOCURRENTS.INFO
The past year of the Covid-19 pandemic has been driven by variants, now designated as Alpha, Beta, Gamma, Delta, and now Omicron. It is natural to wonder what will be next. Scientists and epidemiologists around the world are alert to the possibility that yet another dangerous variant may arise. This is the context to understand the interest in a recent report of a new variant, B.1.640.2, in France.
A variant originally designated B.1.640 was first discovered in southern France in late October 2021. It quickly spread to other areas of Europe, including the United Kingdom, and sections of Africa, including Cameroon and the Republic of Congo.
In early December, B.1.640 was divided into two distinct sublineages: B.1.640.1 and B.1.640.2. The first of these, B.1.640.1 is nearly identical to the original, apart from a 3’ end deletion which we will discuss later on. B.1 640.2 shares a large subset of mutations with B.1.640.1. However, they differ sufficiently that it is likely they both are independent descendants of an unidentified common precursor which we call B.1.640-pre (Figure 1).
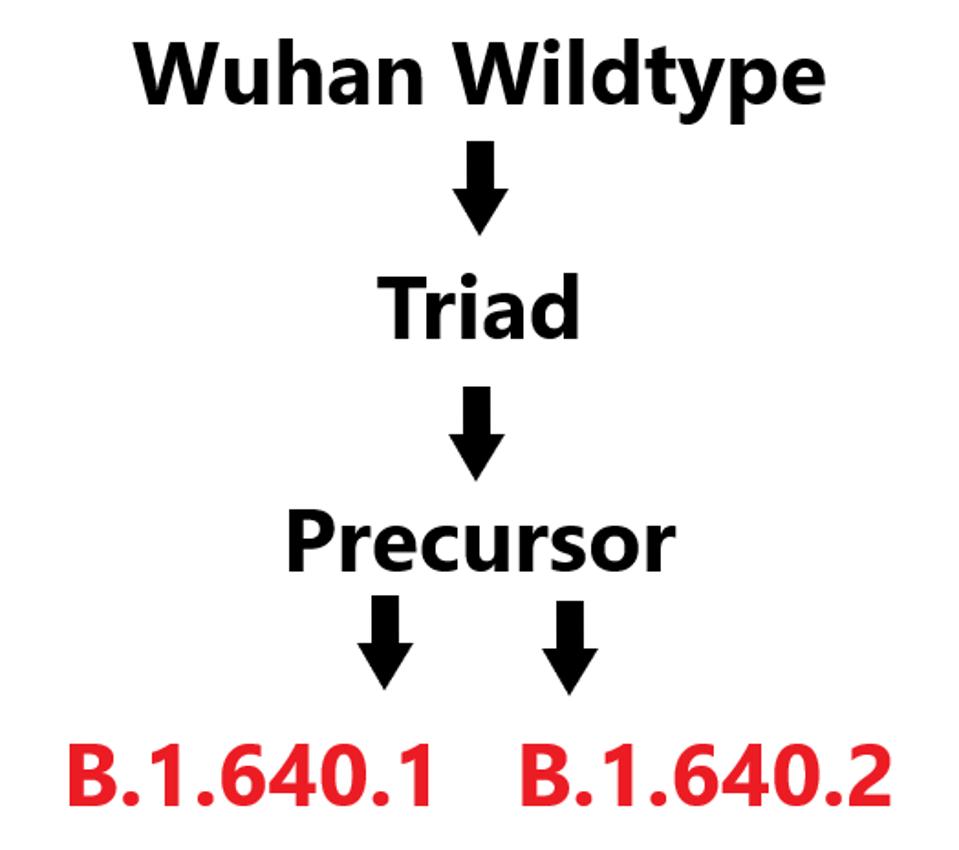
FIGURE 1: Hypothesized lineage of SARS-CoV-2 variants B.1.640.1 and B.1.640.2.
ACCESS HEALTH INTERNATIONAL
When compared to the Omicron variant, these two sublineages share a few important mutations but contain many notable differences, many of which are unique.
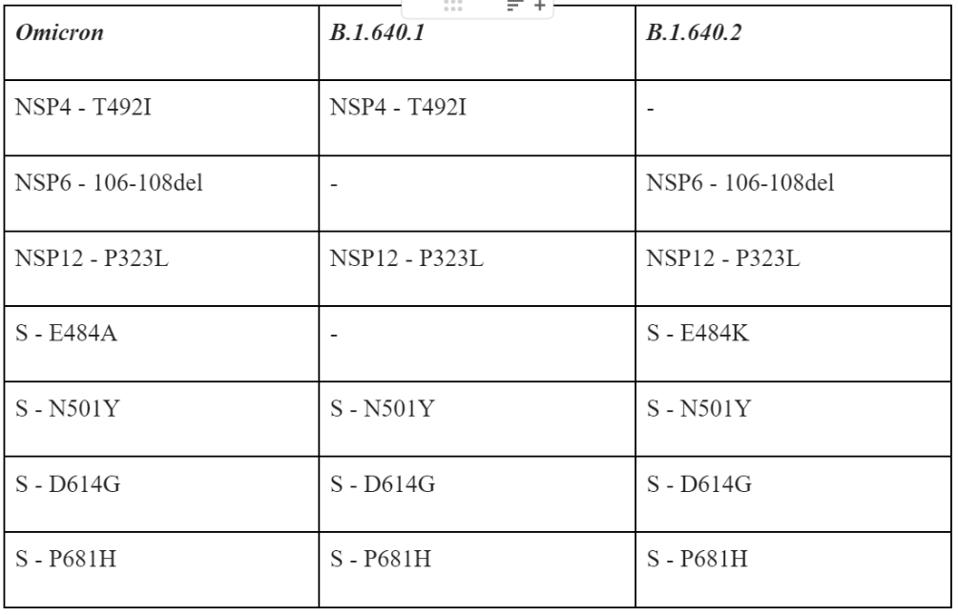
TABLE 1: Similar Mutations between Omicron and the B.1.640 sublineage variants.
ACCESS HEALTH INTERNATIONAL
Table 1 summarizes some of the salient mutations in the B.1 640 viruses that are cause for concern, specifically those they share with Omicron.
Spike Protein Mutations
The 640.1 and 640.2 variants carry 14 and 15 mutations in the Spike protein, respectively, as compared to the Wuhan isolates (Figure 2).
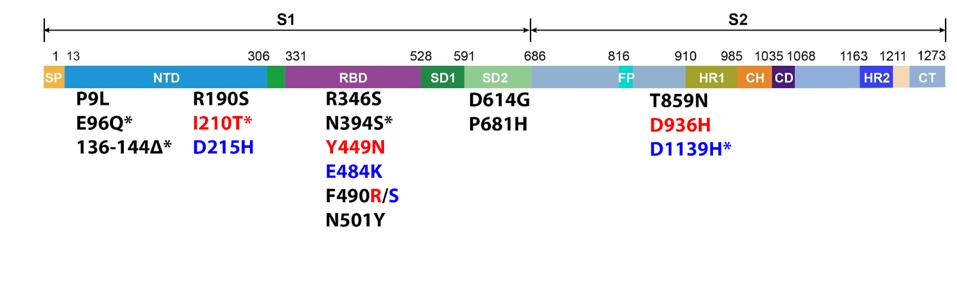
FIGURE 2: Spike protein amino acid mutations in B.1.640 sublineages. Mutations in black are in both B.1.640.1 and B.1.640.2. Mutations in red are distinct to B.1.640.1 and those in blue to B.1.640.2. Mutations with an asterisk are unique to each
ACCESS HEALTH INTERNATIONAL
The eight amino acid deletion, 136 to 144, is present in both 640 sublineages in the N terminal domains, which is likely to remove a dominant neutralizing epitope.
The glutamic acid to lysine mutation at position 484 (E484K) and asparagine to tyrosine mutation at position 501 (N501Y) are common to many variants of interest and reduce neutralization and increase the binding affinity of the Spike protein for the ACE2 receptor. The phenylalanine to arginine/serine mutation at position 490 (F490R/S) and tyrosine to asparagine mutation at position 449 (Y449N) may also increase the affinity of the Spike for ACE2.
Orf1ab Mutations
Non-Spike proteins may also play a significant role in viral pathogenesis, replication, and virulence. Figure 3 summarizes the mutations in the replicative proteins (Orf1ab), the structural proteins other than the spike, namely the membrane protein (M), the envelope protein (E), and the nucleocapsid protein (N), and the accessory regulatory proteins (Orf3a-Orf10).
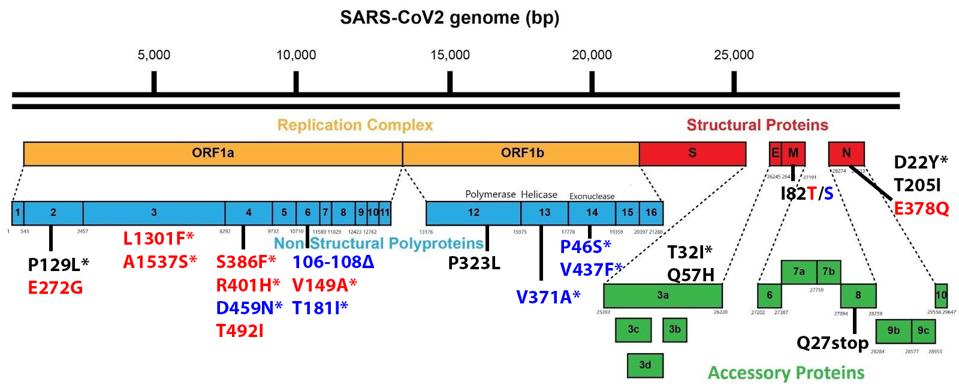
FIGURE 3: Genomic amino acid mutations in B.1.640 sublineages. Mutations in black are in both B.1.640.1 and B.1.640.2. Mutations in red are distinct to B.1.640.1 and those in blue to B.1.640.2. Mutations with an asterisk are unique to the B.1.640
ACCESS HEALTH INTERNATIONAL
There are a large number (15 in total) of mutations in orf1a of the 640 variants. The deletion of three amino acids of NSP6, 106 to 108 is common to other variants of interest and concern.
Structural and Regulatory Protein Mutations
The remaining proteins have a role in the pathogenesis and virulence of SARS-CoV-2. These are the structural and regulatory proteins. While mutations in these regions for the sublineages are relatively limited, their impact may be great.
One mutation of note is in Orf8, which is a rather unusual protein. It plays a role in the inhibition of the innate immune system but is often deleted in SARS-CoV-2 variants, as it is in both sublineages. Residue Q27 is mutated to a stop codon.
The Nucleocapsid threonine to isoleucine mutation at position 205 (T205I) is notable. Recent studies of the protein demonstrate that mutations in the nucleocapsid at this position can increase the titer of virus in culture more than 100 fold. This study indicates that T205I at least doubles transmissibility from the Wuhan wildtype N protein.
Noncoding Mutations
Figure 4 summarizes changes in the nucleic acid sequence that do not alter viral proteins. Recent studies indicate that such subtle changes in the RNA sequence may dramatically affect the abundance of specific virus messenger RNAs and proteins.
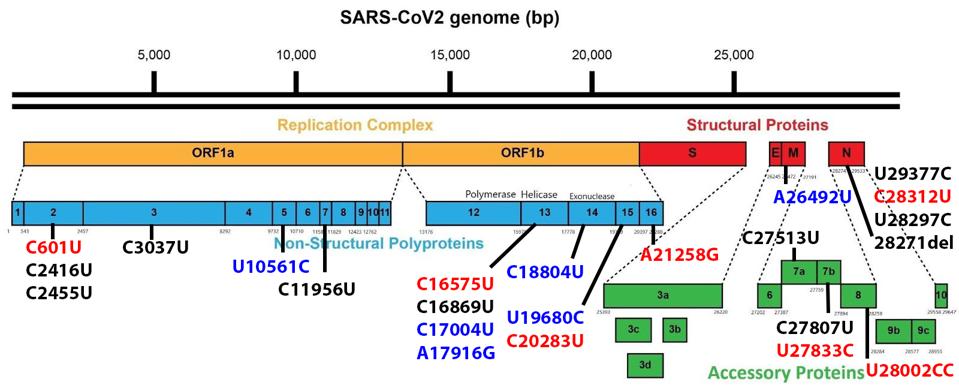
FIGURE 4: Noncoding mutations which do not change an amino acid in the B.1.640 sublineage genome. Mutations in black are in both B.1.640.1 and B.1.640.2. Mutations in red are distinct to B.1.640.1 and those in blue to B.1.640.2.
ACCESS HEALTH INTERNATIONAL
Untranslated Region Mutations
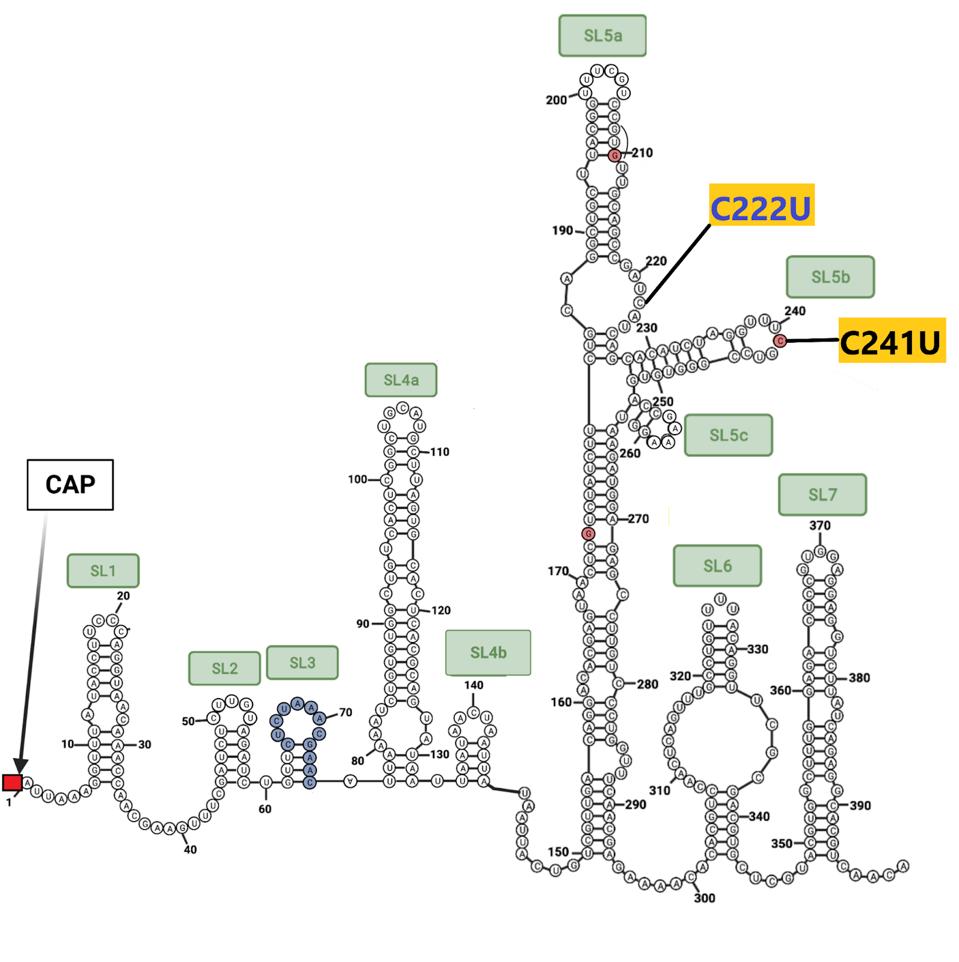
The sequences at the extreme ends of the genome, the 5’ and 3’UTRs, play a central role in viral replication, messenger RNA synthesis, and genome stability. Both 640 sublineages contain the canonical C241U mutation present in one of the earliest variants identified in early 2020. B.1.640.2 contains an additional mutation: C222U. Both lie in stem-loop 5 (Figure 5). The B.1.640.1 sublineage contains an interesting deletion of 20 nucleotides in the 3’UTR, illustrated below.
ACCESS HEALTH INTERNATIONAL
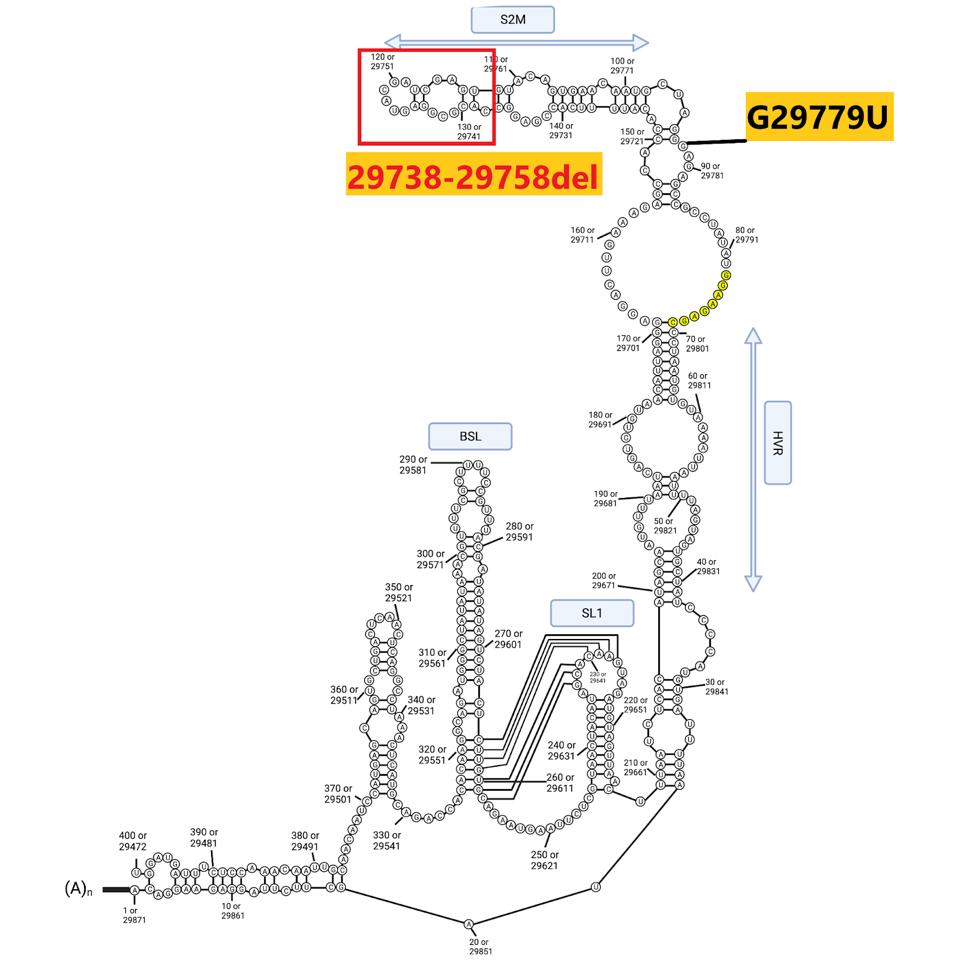
FIGURE 5: 5’ and 3’ end mutations in the B.1.640 sublineages. Mutations in black are in both B.1.640.1 and B.1.640.2. Mutations in red are distinct to B.1.640.1 and those in blue to B.1.640.2.
ACCESS HEALTH INTERNATIONAL
It is too early to know if the B.1.640 variants pose a significant threat. Their significance lies mostly in their existence. It is now clear that SARS-CoV-2 variants pose an ever-present pandemic danger. We are amply forewarned to isolate, characterize, and understand the potential threat that each new variant poses.

