Novel Antiviral Approach To Covid-19 Treatment
(Posted on Thursday, January 20, 2022)
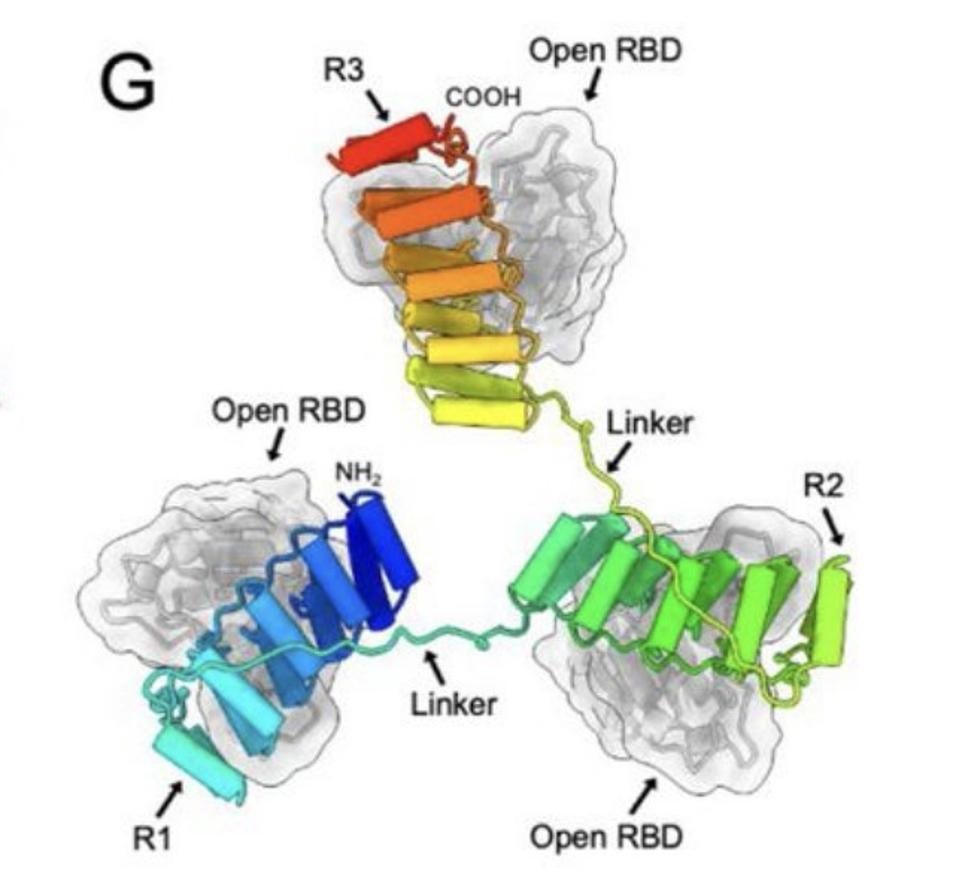
Model of the three RBD-targeting DARPin molecules of ensovibep bound to the RBD regions of the Spike protein.
FROM: “ENSOVIBEP, A NOVEL TRISPECIFIC DARPIN CANDIDATE THAT PROTECTS AGAINST SARS-COV-2 VARIANTS” SYLVIA ROTHENBERGER ET AL. 2021.
As we move into the third year of the Covid-19 pandemic, things continue to look fairly bleak. Caution fatigue combined with the idea that omicron is “mild” have left us with record case numbers and record hospitalizations. But on the treatment front, we’re still lagging, with omicron and other variants resistant to many of our currently available antiviral therapies. That said, Swiss pharmaceutical corporation Novartis, working together with biotech group Molecular Partners, has just thrown its hat in the ring, and early trial results for their new drug, ensovibep, seem promising.
Coming off the back of a successful phase 1 safety study in March of 2021, Novartis and Molecular Partners initiated a global clinical trial called EMPATHY. This phase 2 and 3 study aims to test the safety and efficacy of ensovibep in symptomatic Covid-19 patients in a non-hospitalized setting. Ensovibep is administered as a single-dose intravenous infusion.
The latest results come from phase 2, which focused on preliminary efficacy and on determining the optimal dose. To this end, 407 adult patients were recruited and randomized into four different arms: one for each dose tested, and one placebo control group. All patients displayed at least two symptoms of mild to moderate Covid-19 —fever, cough, body aches, shortness of breath, etc.— and represented a mix of both vaccinated and unvaccinated individuals. Their Covid-19 status was confirmed via positive rapid antigen test the day of dosing as well as a further PCR test at baseline. Dosing took place within seven days of initial symptom onset.
The trial met its primary endpoint, showing a marked reduction in viral load across the span of eight days. It also met the secondary endpoint, with a 78% reduction in hospitalizations, visits to the emergency room, or death when compared to the placebo group. This held true across all of the doses tested: 75mg, 225mg and 600mg. Importantly, all three doses were described as being well-tolerated by the patients, offering a good safety profile. Novartis plans on moving forward with the 75mg dose.
After the results of the first trial phase came through in May of 2021, Patrick Amstutz, Chief Executive Officer of Molecular Partners, said: “By virtue of its tri-specific design, ensovibep was built to resist viral mutations and indeed shows potent inhibition of all variants of concern to date, with the potential to maintain activity also for future variants.”
Mechanism of Action
Ensovibep is based on designed ankyrin repeat proteins (DARPins): genetically engineered proteins that mimic an antibody’s ability to bind to, and eventually neutralize, antigens. DARPins are derived from natural ankyrin repeat proteins, a class of binding protein very frequently found in nature. They can be designed around their exact target and their intended effect, allowing for a great deal of specificity. This results in a high-strength, tight-fitting bond with their target molecule.
In the case of ensovibep, there are five different DARPin domains linked together as one single molecule (FIGURE 1A); two of these bind to human serum albumin (HSA) in order to keep the drug in the body for longer, the other three domains map onto the three-part structure of the SARS-CoV-2 Spike protein. To be more precise, the DARPins simultaneously block the receptor binding domain (RBD) of each spike trimer, allowing them to inhibit both tightly and cooperatively ACE2 interaction (FIGURE 1G). By blocking ACE2 interaction, ensovibep prevents SARS-CoV-2 from binding to our cells and, in so doing, protects them from infection.
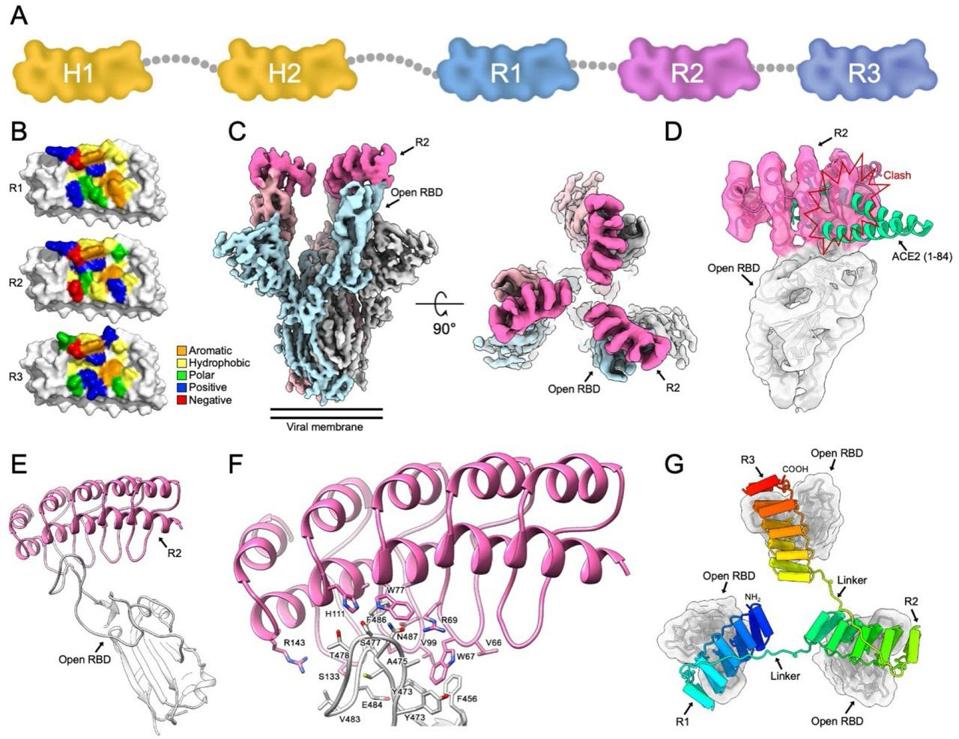
FIGURE 1. A) Schematic representation of the five-part ensovibep construct (half life extenders, H1 and H2, marked in yellow). G) Model of three DARPin molecules bound to the RBD regions of the Spike protein.
SYLVIA ROTHENBERGER ET AL. 2021.
The three-part structure of ensovibep, combined with the high binding affinity of DARPins, makes it difficult for mutations to buck off. The only mutations that managed to do so, a substitution at F486V and another at N234Q, come at the expense of viral fitness more generally. For example, with the F486V substitution, there was a ~8.5 fold reduction in binding affinity between human ACE2 and the SARS-CoV-2 RBD. That kind of reduction would significantly reduce the virus’ ability to infect our cells, decreasing transmissibility and virulence. The importance of F486 is further reflected by a low frequency of naturally occurring substitutions.
In addition to ensovibep’s potential to hold out against variants, it has the benefit of being heat stable —meaning it can be stored and transported easily. Also, because of DARPins’ relatively simple molecular architecture, they can be manufactured in high yields, leading to cost-effective and scalable production.
The main drawback is that ensovibep has to be delivered intravenously, restricting its use to a clinical setting and likely raising its cost. It also means it can’t be used prophylactically, to prevent infection and to prevent transmission, which, realistically, is what we most need to control this pandemic.
We’ll have to wait and see what picture the results from phase 3 paint —which will include a sample size of 2100 patients and double down on testing the clinical efficacy at 75mg— but so far it’s looking like ensovibep will make a valuable addition to our still-sparse quiver of antiviral drugs.

