Covid-19, Gender And Immune Response: What’s The Relationship? (Part Two)
(Posted on Monday, August 8, 2022)
This is the second installment in a two part series which analyzes biological sex differences in immune responses to SARS-CoV-2 infection. Part one focuses primarily on Covid-19 related viral entry, innate and adaptive immune responses to Covid-19 and their correlation to epidemiological evidence. This article will highlight the role of sex hormones in SARS-CoV-2 immune responses, examine sex differences in response to vaccines, and consider their possible therapeutic implications.
Paper cut out illustration of a man and a woman facing each other
GETTY
Covid-19 disease severity and mortality differ between men and women, but the reasons for such differences are not well understood. Part one of this series delves into sex differences in response to SARS-CoV-2 infection and notes how stronger immune responses seen in females likely contribute to the better outcomes observed. This second and final installment will analyze two more elucidating factors: the role of sex hormones on SARS-CoV-2 immune responses and sex differences in immune responses to vaccines. These components, in particular, pose potential therapeutic directions for treating and understanding Covid-19.
Sex Hormones and SARS-CoV-2 Immune Responses
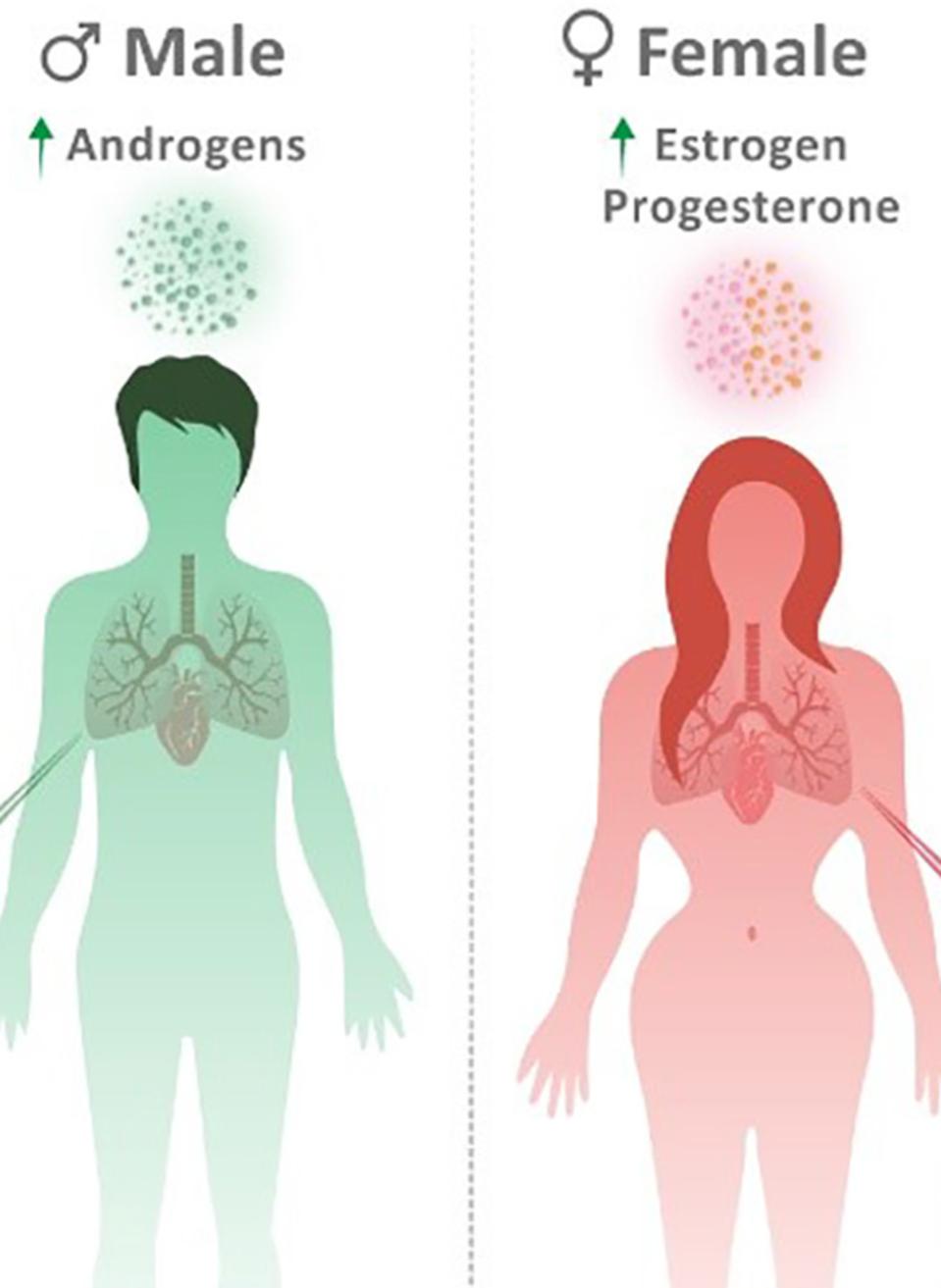
Androgens
In their review The Immune Response to Covid-19: Does sex matter?, Ho et al. analyze the complex relationship between sex hormones and SARS-CoV-2 immune response. They first consider androgens—such as testosterone and dihydrotestosterone—which males possess higher levels of than females.
Ho et al. find that androgen receptor expression may impact two essential enzymes to SARS-CoV-2 viral entry: furin and transmembrane serine protease 2 (TMPRSS2). Furin is a calcium-dependent enzyme which cleaves the spike protein into the configuration needed for priming and activation. Transmembrane serine protease 2 (TMPRSS2) primes the SARS-CoV-2 protein for entry into host cells. The theory is, since increased androgen receptor expression can upregulate furin and TMPRSS2, the higher androgen receptor expression seen in men increases their susceptibility to severe forms of Covid-19.
Although the clinical association observed between androgenic alopecia and severe Covid-19 would suggest this mechanism to be true, studies on androgen deprivation therapy (ADT) in prostate cancer patients with Covid-19 do not necessarily support this claim. Androgen deprivation therapy reduces the number of androgen receptors available for activation through medicine or surgery. The therapy was expected to decrease androgen receptor expression in prostate cancer patients with Covid-19, thereby restricting androgen regulation of TMPRSS2 and reducing the risk of SARS-CoV-2 infection. In contrast to this notion, the treatment did not improve infection risk, ICU admission, hospitalization or mortality in comparison to controls.
Randomized clinical trial results with antiandrogens, medicines which block androgen receptors and inhibit androgen synthesis, further complicate these associations. One randomized controlled trial revealed that Covid-19 patients given nitazoxanide/azithromycin therapy with antiandrogen dutasteride experienced decreased viral shedding, inflammatory markers and time-to-remission compared with placebo; another found that antiandrogen proxoludamine reduced the 30-day hospitalization rate and risk ratio amongst men with Covid-19. On the other hand, a third trial with enzalutamide increased Covid-19 related hospitalization stay.
Male sex steroids seem to perform varying roles with respect to Covid-19. The culminating conclusion from these studies suggests that both low and high androgen levels can correlate with poor Covid-19 prognoses. As Ho et al. state in their review, further investigation in this arena is needed.
Estrogens
Female sex hormone estrogen appears to mediate several beneficial immune responses. A study of hospitalized Covid patients correlated higher estradiol levels to decreased disease severity. And as mentioned in part one, estrogen promotes strong immune responses in women and likely contributes to the observed discrepancy in innate and adaptive immune responses between sexes.
Inflammation in female innate immune responses reduces when estrogen activates anti-inflammatory cytokines, inhibits the nuclear factor kappa B (NF-B) pathway, and decreases the release of inflammatory cytokines. Women also have better priming of adaptive immune responses to viruses. This is thought to be influenced by estrogen; estrogen can help regulate immune cells called plasmacytoid dendritic cells (pDCs) which, in turn, promote the production of interferon alpha, an important antiviral cytokine in innate immunity. These mechanisms may translate to the better disease outcomes witnessed in women than men with Covid-19.
Estrogen has also been found to regulate several proteins which are involved in SARS-CoV-2 viral entry: furin, TMPRSS2, angiotensin converting enzyme 2 (ACE2) and a disintegrin and metalloprotease 17 (ADAM17). It, too, suppresses immune enzyme dipeptidyl peptidase 4 (DPP4), thereby blocking another potential means of SARS-CoV-2 viral entry.
Researchers are exploring possible therapeutic applications for estrogen in Covid-19 interventions. Two examples include a study on the effect of selective estrogen receptor modulators on Covid-19, and a randomized control trial analyzing the efficacy of an estradiol/progesterone therapy in reducing disease severity in hospitalized Covid patients.
Progesterone
Ho et al. complete their study of sex hormones in Covid-19 with progesterone. Progesterone levels tend to be higher in women than men and are associated with general anti-inflammatory effects. These anti-inflammatory effects include but are not limited to the ability to increase T regulatory cells, enhance antiviral immune pathways and disrupt endocytic pathways used by viruses to enter host cells. It is hypothesized, therefore, that progesterone may decrease the risk of hyperinflammation and SARS-CoV-2 related cytokine storm.
There is therapeutic potential in administering progesterone to treat Covid-19. A study of hypoxemic men hospitalized with Covid-19 observed that short term subcutaneous progesterone decreased hospitalization stay and supplemental oxygen needed. Additional research is needed to understand the specific mechanisms at work and its promising impacts on Covid-19 treatments.
Sex Differences in Vaccine Immune Responses
Vaccines are crucial to Covid-19 control and have been invaluable in reducing lives lost to severe forms of the disease. As a result, Ho et al. emphasize the importance of understanding sex differences in response to Covid-19 vaccines. They state, “sex differences should be taken into account as a biological variable for adjusting sex-personalized vaccine dosage and considering vaccine efficiency.”
These considerations seem most pertinent to women. Two studies, one systematic review and one meta analysis, found that vaccination prevented Covid-19 disease less effectively in women than in men. Similarly, a 2021 CDC report observed that women received 61% of administered Covid-19 vaccines at the time yet accounted for 79% of adverse events. The discrepancies in vaccine response could be due to several factors—age, hormonal differences (as explored in this article) and sex differences intrinsic to SARS-CoV-2 immune response (see part one of this series)—but more studies are needed to clarify these possible correlations.
Conclusions
Contemporary research reveals that sex hormones and biological sex do influence immune responses and vaccines, although specific mechanisms have yet to be fully understood. Ho et al. call for biological sex to be considered in basic, translational and clinical Covid-19 research. More extensive research on biological sex and Covid-19 could open potential therapeutic avenues and improve the specificity of those strategies—be it through the use of sex hormone therapies or through the adjustment of vaccine dosage based on gender.

