Why The Covid Vaccines Work And How To Make Them Better
(Posted on Wednesday, December 7, 2022)
It’s no wonder that by now, people are looking both to understand how the Covid vaccines work and to determine how best to improve them. Current mRNA vaccines are excellent at raising high neutralizing-antibody titers against the strain to which they were designed, namely the early Wuhan strain. However, antibody levels fade quickly after three to four months, yielding poor effectivity even against the original strain. Immunity quickly fades after vaccinations, boosters, and even after vaccination with the recent bivalent boosters. Furthermore, the original vaccines and the updated booster generate weak neutralizing activity against the latest variants. Bowen et al. have undertaken a systematic study to understand why the vaccines seem to work as well or poorly as they do and with the thought that they may be able to improve their activity.
Background
The mRNA vaccines that most Americans received over the past year and a half are prefusion spike protein from the SARS-CoV-2. When the virus infects a host, the S1 portion of the spike locks onto a host cell, and the S2 portion engages fusion mechanisms after undergoing a conformation shift. The vaccines teach the natural immune system to identify the spike and neutralize it before cell infection occurs.
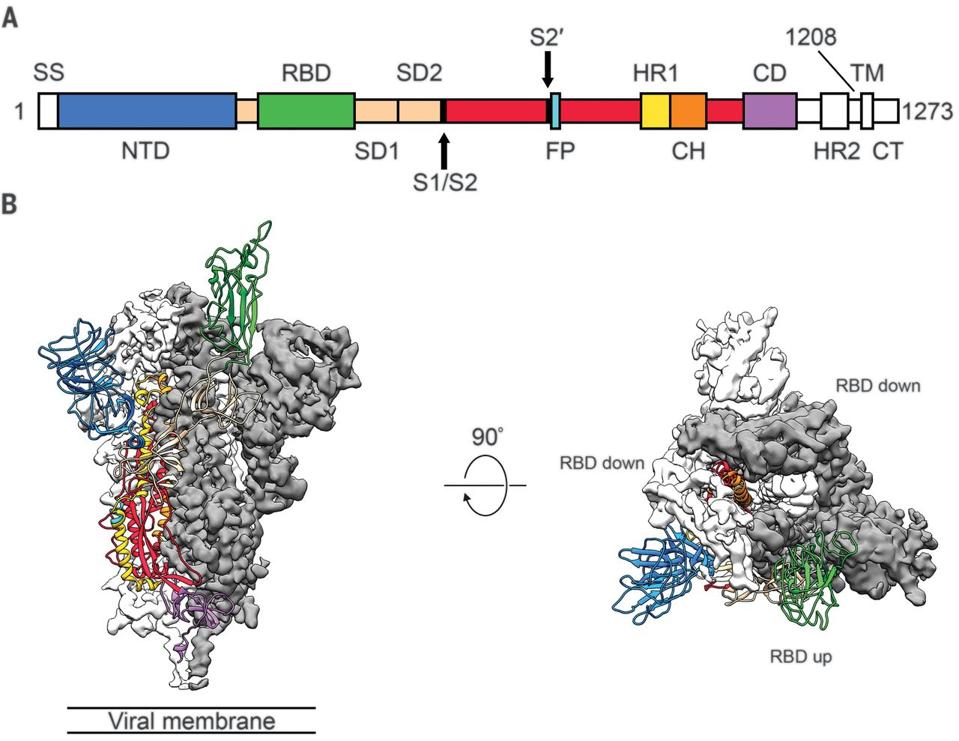
FIGURE 1: Structure of 2019-nCoV S in the prefusion conformation. (A) Schematic of 2019-nCoV S primary structure colored by domain. Domains that were excluded from the ectodomain expression construct or could not be visualized in the final map are co
WRAPP ET AL.
However, as the virus mutated over time in the form of variants such as Alpha, Delta, and now Omicron and its sublineages, vaccines became less and less effective at creating antibodies that recognize the modified spike.
Researchers Bowen et al. from the Veesler lab at the University of Washington aimed to evaluate the influence of spike conformation on plasma-neutralizing activity through a series of spike protein experiments. In other words, where is the best target for vaccine-elicited antibodies, and during what conformation should those antibodies strike?
Prefusion SARS-CoV-2 S stabilization reduces the fraction of antibodies recognizing an off-target conformational state
To determine the importance of proline stabilization, the researchers generated in vitro models of the entire spike, the S1 portion containing the N-terminal domain and receptor-binding domain, the S2 portion pre-fusion, and the S2 portion post-fusion. Proline stabilization refers to inserting two prolines into a pivotal joint of the vaccine’s spike protein to stabilize the structure’s perfusion shape. The three that include the ‘2P’ mutation are Moderna’s mRNA-1273, Pfizer’s BNT162b2, and Novavax’s NVX-CoV2373.
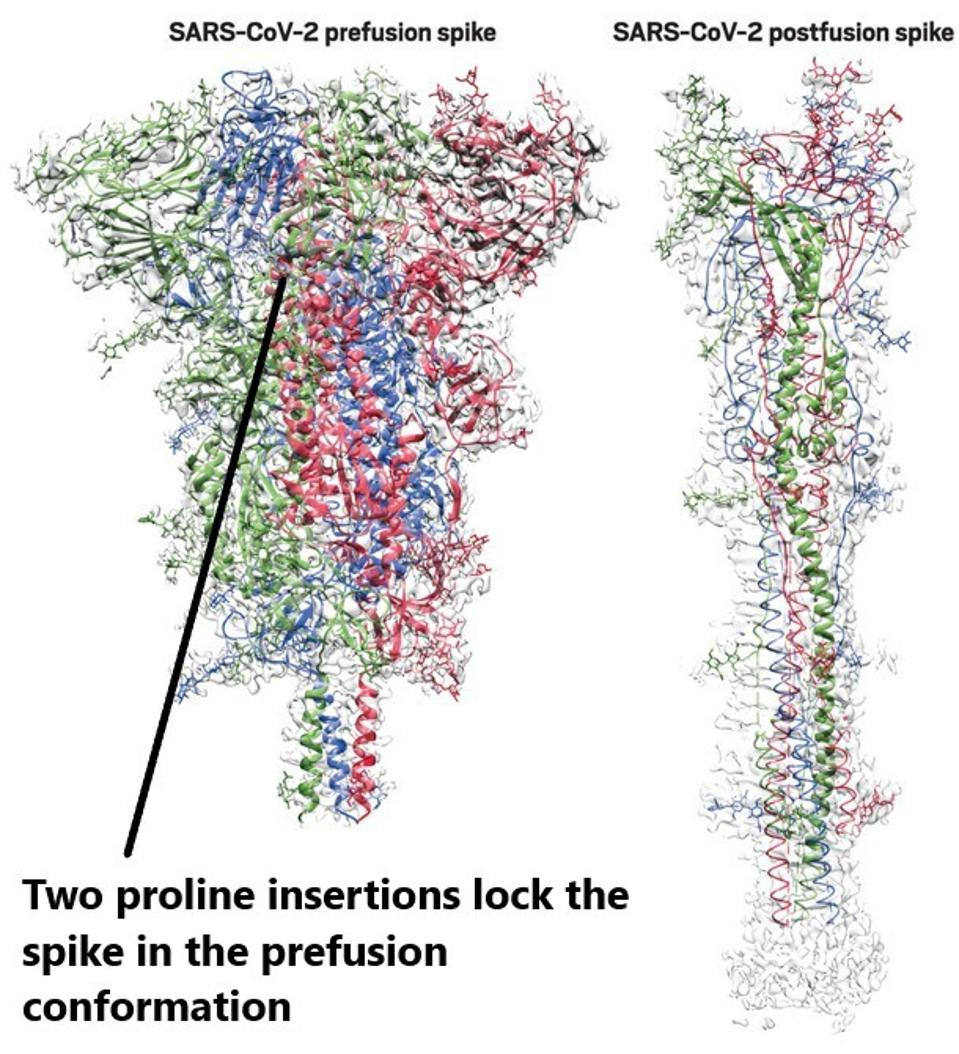
FIGURE 2: Prefusion and postfusion structures of the SARS-CoV-2 spike protein. The spike sheds a subunit and elongates during fusion with a human cell. Proline stabilization denoted.
BING CHEN
They gathered sera from a wide panel of subjects vaccinated with different vaccines, all of which were not infected before vaccination. The researchers introduced the differing sera to the different in vitro spike targets.
Six of the seven vaccines examined yielded much stronger neutralizing activity against the whole spike and the S1 subunit. The lone exception was BIBP-CorV, an inactivated SARS-CoV-2 vaccine by Sinopharm.
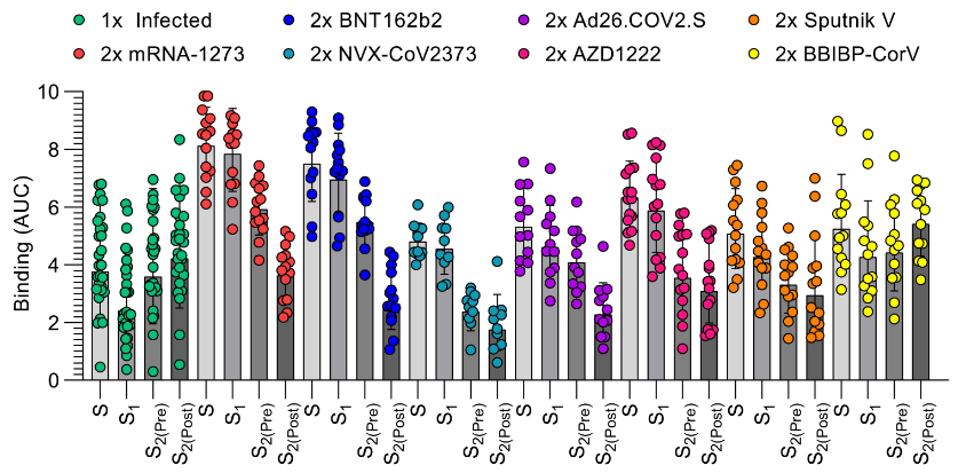
FIGURE 3: IgG binding titers elicited by SARS-CoV-2 infection or vaccination against prefusion S (S), the S1 subunit, and the S2 subunit in the prefusion (S2(Pre)) and postfusion (S2(Post)) conformations.
BOWEN ET AL.
Bowen et al. note that proline-stabilized vaccines are among the most robust in S1 targeting and neutralization compared to S2 pre- and post-fusion.
SARS-CoV-2 neutralization is determined by S1 subunit targeting antibodies
Next, Bowen et al. affirm the necessity of S1 subunit targeting antibodies for virus neutralization. The researchers sorted the antibodies found in the sera of subjects who received Moderna or Pfizer’s mRNA vaccines by the binding site. After sorting, they could then deplete specific antibodies by spike target, whether perfusion spike, S1, prefusion S2, or postfusion S2.
The results demonstrate that the prefusion spike and S1 are by far the most crucial to antibody neutralization of the virus. When those antibodies were depleted, neutralization fell dramatically, whereas when S2 pre- or post-fusion was depleted, neutralization remained roughly the same.
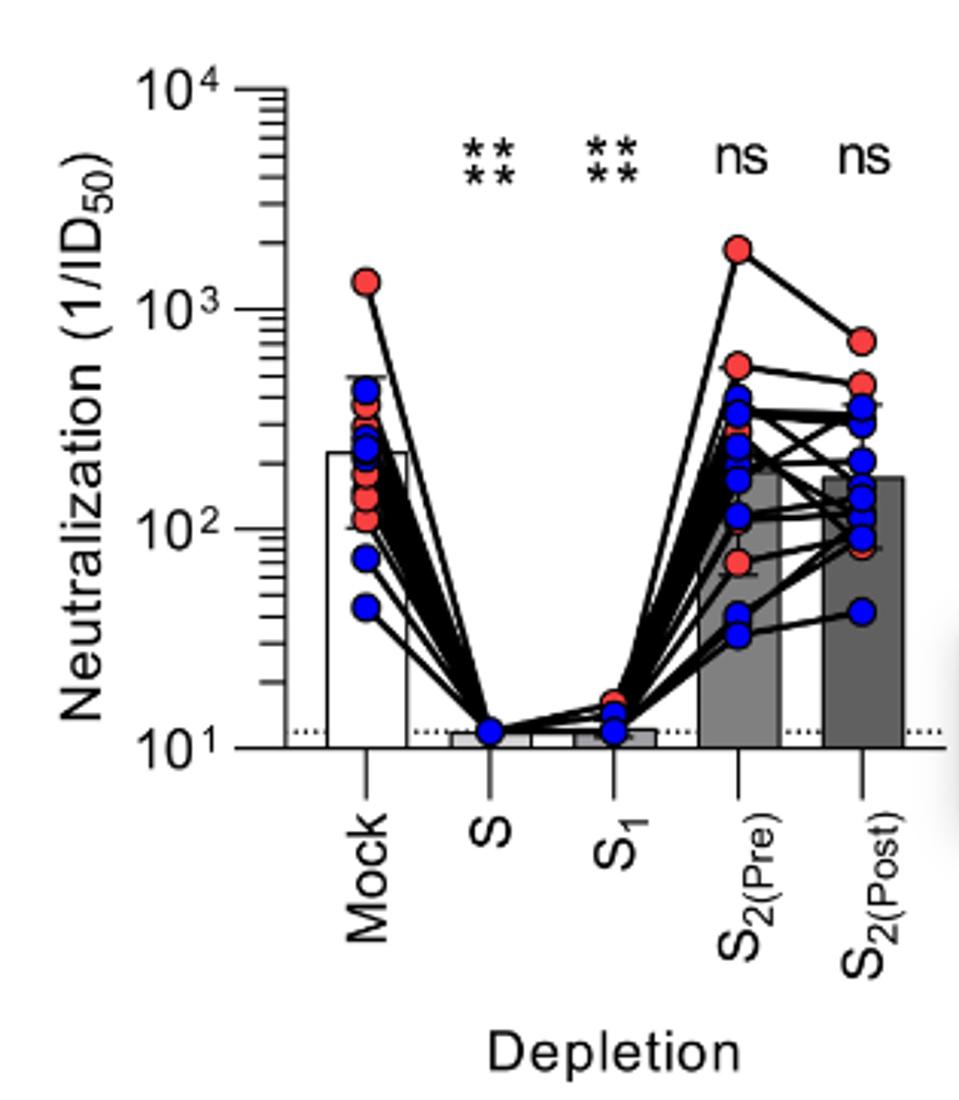
FIGURE 4: Neutralization titers resulting from depletion of polyclonal plasma antibodies targeting S, S1, prefusion S2, and postfusion S2.
BOWEN ET AL.
What does this tell us about vaccines? They are most strongly neutralizing when introduced to the S1 subunit, namely the receptor-binding and N-terminal domains. Not surprisingly, these are the regions most heavily mutated in Omicron and its sublineages.
SARS-CoV-2 variant cross-neutralization is determined by RBD-specific antibodies
Finally, Bowen et al. show that the N-terminal domain is nearly inconsequential in terms of antibody neutralization. They conducted the same depletion experiment, this time comparing the N-terminal and receptor-binding domain targeting antibodies of the S1 subunit group.
Upon depletion of the receptor-binding domain targeting antibodies against major variants, neutralization drops to near undetectable levels. The opposite holds true for N-terminal domain antibodies.
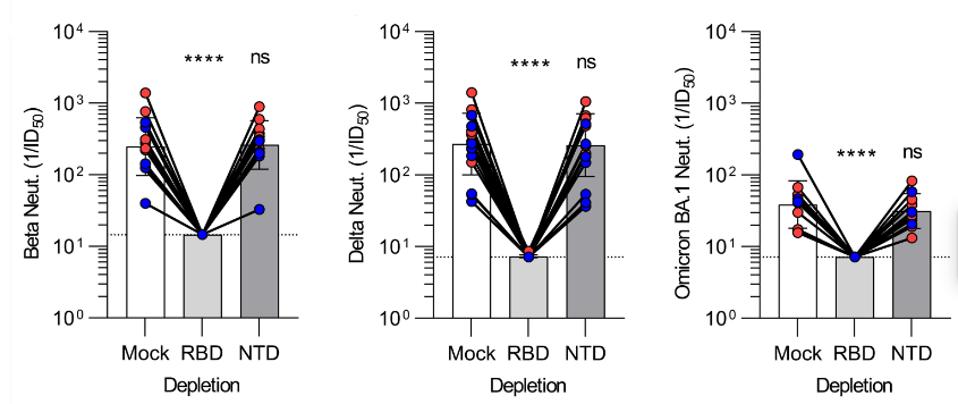
FIGURE 5: Plasma neutralizing activity against Beta S VSV, Delta S VSV, and Omicron BA.1 S VSV after mock, Wuhan-Hu-1 RBD, or Wuhan-Hu-1 NTD depletion of polyclonal antibodies.
BOWEN ET AL.
This tells us that N-terminal domain targeting antibodies elicited from vaccines are inconsequential in the neutralization of SARS-CoV-2 and its variants. Paired with our newfound knowledge that S2-targeting antibodies are also far less involved in neutralization, we can conclude that receptor-binding domain targeting antibodies are our best bet at neutralizing the virus. This confirms our ongoing suspicion that the numerous receptor-binding domain mutations in Omicron and its sublineages are those responsible for the evasion of vaccine protection.
Concluding Thoughts
How can this study inform us of the current status of vaccines and how to move forward? The vaccines released in 2021 by Moderna, Pfizer, and others neutralize Omicron and its sublineages very little to not at all. The updated bivalent vaccines released in 2022 may be slightly better but still fall well short of the necessary mark.
Bowen et al. provide more detail about why the vaccines work as well as they do but offer very little about how you might improve their activity. We need considerably more work to understand what we can do to either prolong immunity or amplify memory. Some are beginning to despair that vaccines alone will not be the answer for the limitation of Covid-19 symptoms and infections. Perhaps shifting focus to a combination of vaccines plus highly active and long-acting antiviral drugs may provide the answer in the long run.

