FDA Authorizes New Antibody Treatment For Severe Covid-19
(Posted on Wednesday, April 12, 2023)
A new antibody drug by pharmaceutical company inflaRx received FDA emergency use authorization to treat critically ill Covid-19 patients. One of the greatest hurdles for monoclonal antibody development during the Covid-19 pandemic is the ongoing mutation of the SARS-CoV-2 virus. Most antibodies introduced throughout the pandemic targeted the virus spike protein, which mutates significantly with every emerging variant. The monoclonal antibody, vilobelimab, does not target the spike protein, nor the virus. Rather, it targets a specific product of the complement system; an immune response that triggers most of the severe symptoms we experience when battling a dangerous pathogen, including inflammation, pain, fatigue, etc.
The antibody recently underwent phase one and two safety and efficacy trials in human volunteers, demonstrating a strong response in controlling severe symptoms. Here we discuss the antibody and its trial results, discussing the potential impacts it may have now that it has been authorized.
Mechanism of Action
The targeted complement molecule for vilobelimab is C5a. The complement system comprises a cascade of molecules C1-C9 activated upon pathogen contact with an antibody. The cascade initiates a series of reactions that destroy infected cells, but in the process, the host must deal with side effects that manifest as symptoms. Less severe cases may include moderate tiredness or physical pain, whereas severe symptoms include inflammation and significant fatigue.
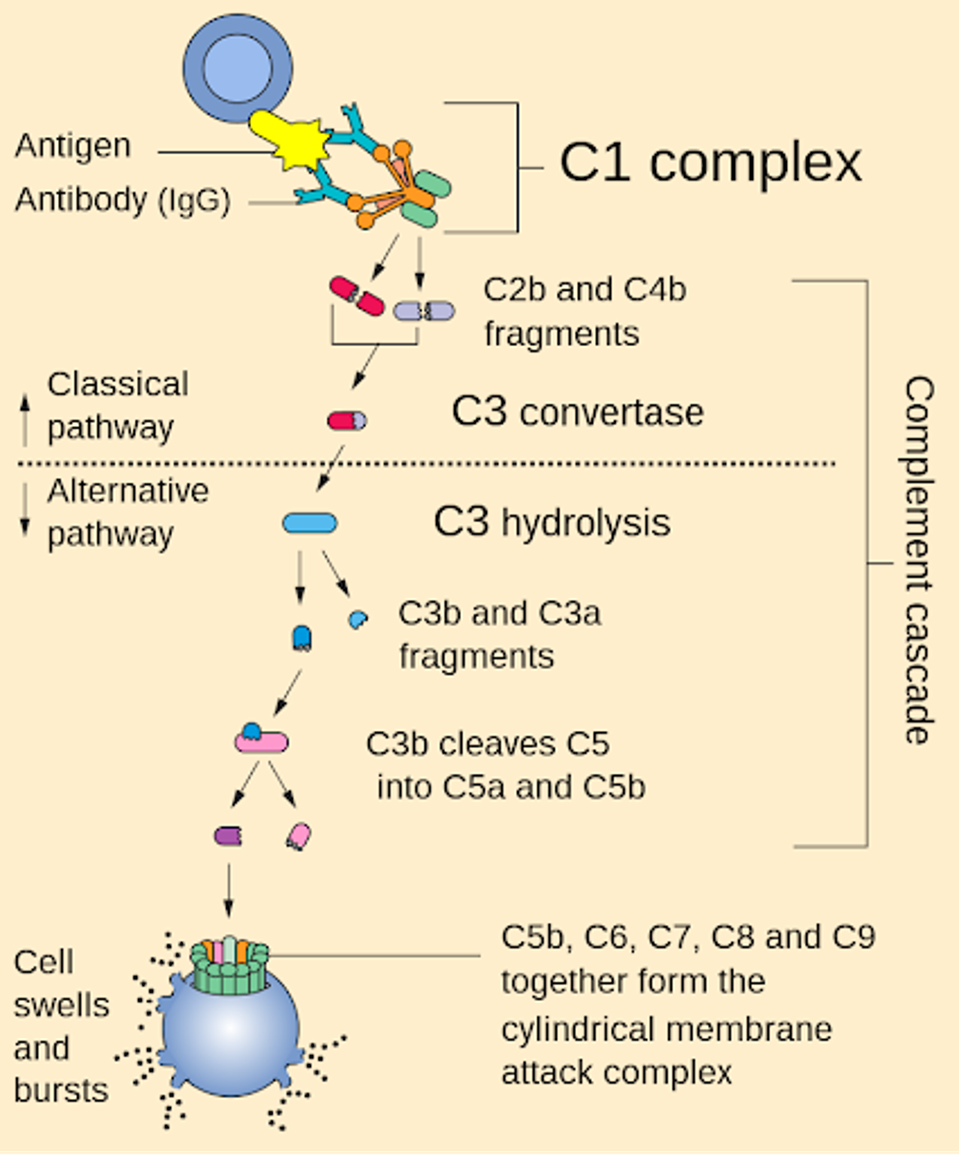
FIGURE 1: Schematic of the immune complement system.
NIH
The inflaRx team focused on one such molecule fragment, C5a, which acts as a chemoattractant for other immune system mechanisms. Once activated, C5a signals other cells, such as macrophages, to attack invaders and infected cells.
They found that a specific epitope within the C5a molecule could be exploited for a potent blocking monoclonal antibody, which could temper the C5a signaling and reduce the severity of complement-related symptoms in severe disease cases.
Their research led to vilobelimab. Originally, the monoclonal antibody was developed to treat hidradenitis suppruativa, or HS; a common skin condition that causes painful lumps deep in the skin. These lumps are a result of hyperinflammation and infection in the sweat glands. An antibody that could reduce inflammation by impeding the complement system could relieve those living with HS. Such reduction in inflammation could also be applied to Covid patients.
The antibody delivered a complete immunological block of C5a-induced effects, resulting in strong therapeutic responses. The drug was also fully selective and has high binding affinity, meaning it did not interfere with related cellular activities such as membrane attack complex, reducing autoimmune complications.
Efficacy Trials
A trial conducted by Vlaar et al. aimed to determine survival outcomes impacted by vilobelimab as a therapeutic. Their phase three trial included 368 patients in nine countries worldwide, all suffering from severe Covid-19 and requiring ventilation.
In the treatment group, 54 patients of 177 (31%) died due to Covid-related health complications within 28 days of treatment, whereas 77 of 191 (40%) died in the control placebo group in the same timeframe.
While modest, the drug did improve survival outcomes for patients by nine percentage points, which considers the drug a suitable therapy for patients with severe Covid-19.
Discussion
Ultimately, vilobelimab is not a miracle cure for Covid-19. The modest reduction in Covid-related death in severe patients is notable but not groundbreaking. Combining vilobelimab with other effective monoclonal antibody treatments may improve the disease outcomes even further, and additional research into this possibility should be explored. Notably, monoclonal antibodies that target highly conserved regions of the spike, such as the S2 region, or anti-ACE2 antibodies we have previously discussed could be possible combination options.

