Adapted SynNotch, A New Generation Of Flexible And Controllable CAR T Therapies (Part 3)
(Posted on Monday, June 5, 2023)
This is the final installment in our series on alternate CAR T therapies. Part one describes a version of CAR T which uses a universal adaptor. Part two introduces SNAPtag, an alternative CAR T therapy. Visit the website www.williamhaseltine.com for more on CAR T therapies.
In a previous installment, we described an alternative cell treatment which relies on synNotch receptors. These synthetic receptors react to a specific input and produce a desired cell response. This modular design promises greater therapeutic flexibility than its more well-established counterpart, CAR T therapy. The design is further improved when integrated with a universal adaptor called SNAPtag. Here, we describe how researchers at the University of Pittsburgh developed this synNotch-adaptor duo and how it works.
SynNotch and the Universal Adaptor
SynNotch cells depend on transcription factors to change a gene, while CAR T cells trigger T cell signal cascades. Despite relying on distinct mechanisms, the two therapies share similar limitations: they are both hard to control and can only target one antigen at a time. To this end, Ruffo and colleagues sought to develop an adaptor that worked not only with CAR T cells, but with synNotch T cells, as well.
The adaptor should, in theory, create a bridge between the receptor and the target antigen. In this way, using an adaptor on synNotch cells would allow the therapy to target multiple antigens at the same time or sequentially. The antigen target could also be switched by introducing a new antibody with the same tag. And importantly for control, the therapy could be halted or intensified by altering the adaptor concentration or introducing tags as a competitive inhibitor.
Universal Adaptor
As demonstrated previously, the universal adaptor is composed of two key elements: an antibody and a benzylguanine (BG) tag (Figure 1). The tag permanently fastens to the extracellular portion of the synNotch receptor via covalent bonding. Meanwhile, the antibody tail senses and binds to a specific antigen on a target cell. Antigen binding stretches the synNotch receptor and reveals a cleavage site on its core protein. As Figure 2 shows, enzymes will cut the cleavage site and release a transcription factor into the intracellular domain of the cell. The transcription factor should travel to the nucleus, where it influences the expression of a target gene.
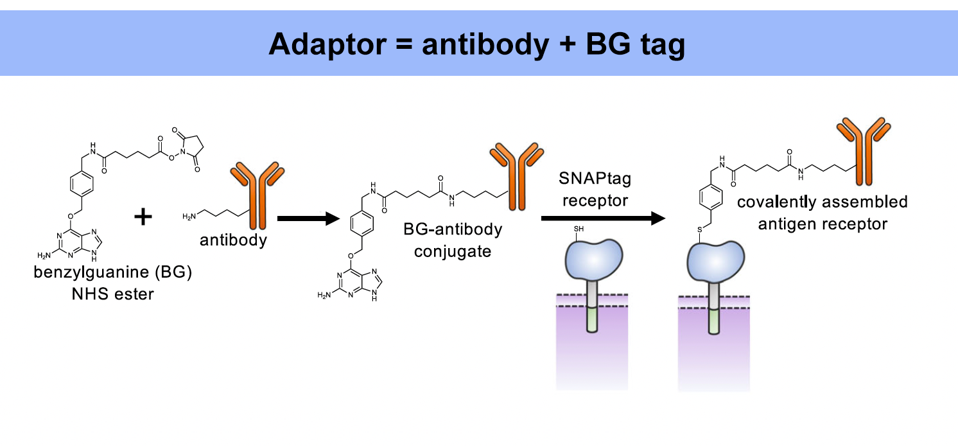
FIGURE 1: The universal adaptor depends on two components: an antibody and a benzylguanine (BG) tag. The tag covalently binds to the synNotch receptor, while the antibody binds to a specific antibody target. The same tag can be fused to a variety of antibodies, allowing the synNotch receptor to respond to a wide assortment of antigen targets.
RUFFO ET AL., 2023.
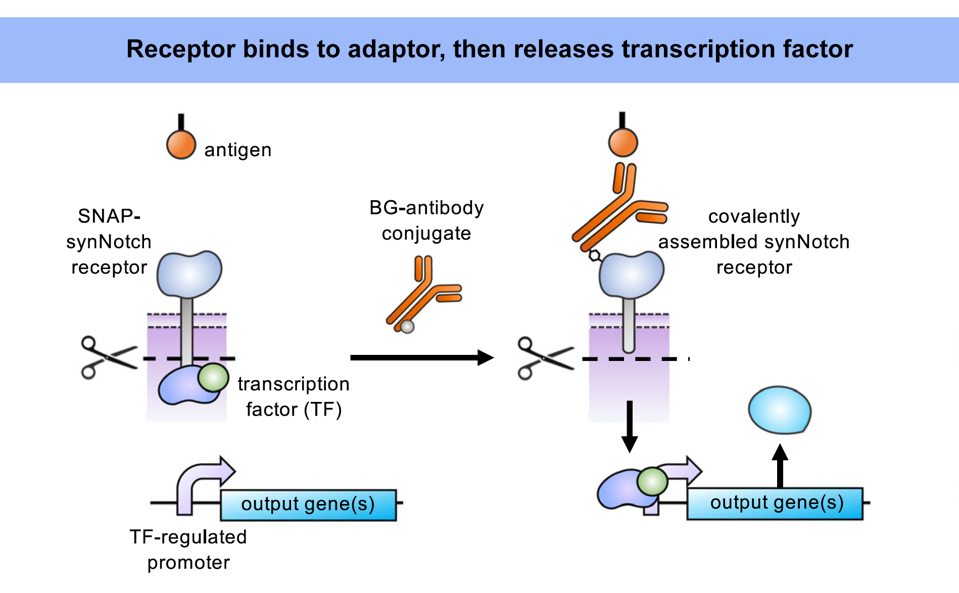
FIGURE 2: SynNotch receptors trigger a different pathway than CAR T cells in the presence of a universal adaptor. When the adaptor tag is bound to the receptor (and the adaptor antibody to the target antigen), mechanical pulling forces stretch the receptor and expose the cleavage sites on the core protein. Cleaving releases the transcription factor and activates a target gene once inside the nucleus.
RUFFO ET AL., 2023.
Does Adapted SynNotch Work?
SynNotch receptor function was tested with four different types of adaptors, each composed of a distinct and clinically relevant antibody (see Table 1). The synNotch cells were co-incubated with corresponding tumor cells and exposed to differing concentrations of the adaptor. After 48 hours, the cells were assayed using flow cytometry to measure gene expression. Target gene expression should increase if the adaptor molecules successfully bind to a tumor cell on one side and a synNotch receptor on the other.
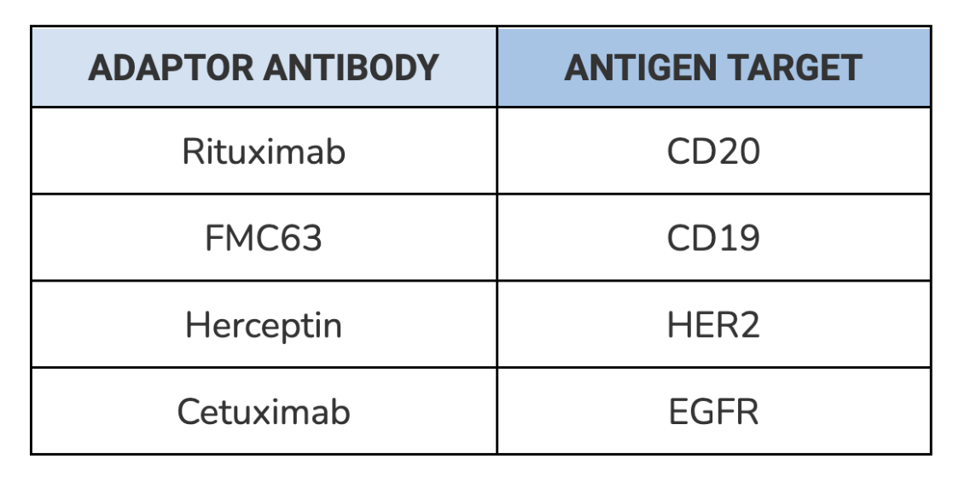
TABLE 1: List of antibodies used to test the universal adaptor. Each antibody targets a specific antigen found on tumor cells.
ACCESS HEALTH INTERNATIONAL
Results
The synNotch cells successfully activated target genes when exposed to the universal adaptor. Notably, the synNotch cells could regulate the expression of a gene called IL-7 which promotes T cell proliferation—an important component to bolster a patient’s immunity.
Receptor activation proved sensitive at low concentrations of the adaptor. Fascinatingly, once the adaptor concentration exceeded 0.25µg/mL, target gene expression no longer increased. In fact, a “hook effect” was observed; although the adaptor concentration increased, target gene expression continually decreased. Gene expression was completely inhibited in adaptor concentrations of 10µg/mL.
The authors posit that the “hook effect” occurred due to oversaturation. Likely at these concentrations, the adaptor molecules are binding to the target tumor or synNotch cells without creating a bridge. Without binding on both sides of the adaptor, the synNotch receptor cannot be activated.
Implications
SynNotch T cells have the potential to exceed CAR T cell performance with their flexibility. The design allows researchers to determine the input and output of a cell response. Integrating a universal adaptor further extends the possibilities of the treatment to respond to multiple antigen targets or turn on/off at will. While presently in early stages of testing, clinical translation of this platform would greatly benefit patients with solid tumors and other hard-to-treat diseases.

