Beyond Paralysis: Advances In Brain-Spine Interfaces For Post-Injury Movement Restoration
(Posted on Monday, June 12, 2023)
Originally published on Forbes on 6/12/2023

Gert-Jan (left) and a scientist walk with his “digital bridge.”
CHUV / GILLES WEBER
The following is part of a series on brain-machine integration and biomechanical solutions to restore function to tissues damaged by disease, trauma, or time.
The latest advances in brain-spine interface technology may help those with impeded lower body motor functions to walk again. Roughly 300,000 Americans today have suffered significant spinal cord injuries, resulting in lower or full-body paralysis. Most of us take the ability to walk for granted, doing so without conscious thought. The movements are so regular that our brain can conduct them without having to actively think about them. Electrical signals from the motor cortex trigger the muscle contractions necessary to move swiftly from point A to point B while maintaining balance.
When a spinal injury results in paralysis, the neural connection between lower body muscles and the motor cortex is damaged or completely severed, leaving many without the ability to walk. While a vast majority of these cases are permanent, recent advances may establish the framework for post-paralysis restoration of movement.
Lorach et al. from the NeuroX Institute in Switzerland developed a brain-spine interface to restore the spinal gap created by injury. The digital bridge would translate neural electrical signals across the gap, resulting in brain-driven muscle movement in the lower body. Here we examine this technology and its implications for regenerative medicine.
Lorach et al.’s brain-spinal interface is comprised of two systems: WIMAGINE and ACTIVA. The brain system, WIMAGINE, is a set of two fully-internal implants positioned directly adjacent to the sensorimotor cortex on each side of the brain. At 50 mm in diameter, the implants are undetectable once integrated within the skull. Accompanying the implants are two external antennas stylized as an external headset. The implants and antenna detect and transfer electrical impulses from the brain’s motor cortex to ACTIVA, the digital bridge embedded in the spine.
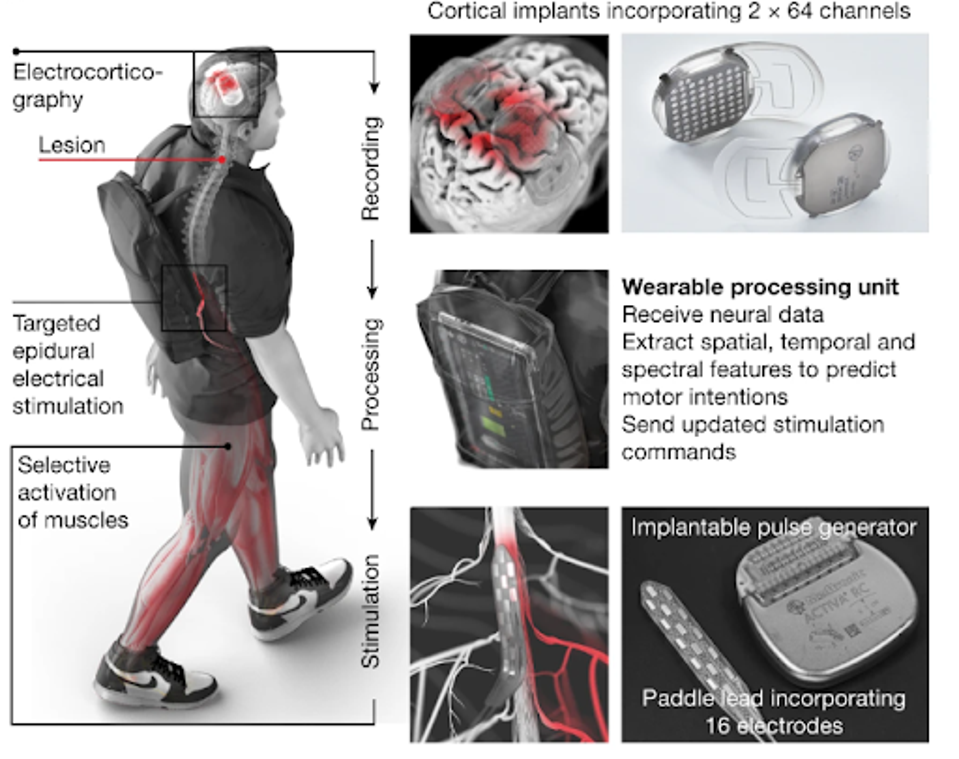
FIGURE 1: Two cortical implants composed of 64 electrodes are positioned epidurally over the sensorimotor cortex to collect ECoG signals. A processing unit predicts motor intentions and translates these predictions into the modulation of epidural electrical stimulation programs targeting the dorsal root entry zones of the lumbosacral spinal cord. Stimulations are delivered by an implantable pulse generator connected to a 16-electrode paddle lead.
LORACH ET AL.
ACTIVA is an implanted pulse generator normally used for brain stimulation in Parkinson’s patients. The wireless spinal implant detects the signals sent from the WIMAGINE antenna, converting them into analog impulses that stimulate the dorsal neurons previously impacted by the injury. The result is near real-time translation of motor-cortex neural firings to lower body muscle movement.
The researchers tested their brain-spinal interface on a tetraplegic who had a biking accident 10 years prior. He had previously been involved in neurorehabilitation, which restored minor motor ability with the help of a front wheel walker, but his recovery had plateaued. The brain and spinal implants were installed, and he was discharged 24 hours after surgery.
The two systems require annual calibration, which consists of the mapping of neural signals in response to asking the patient to make specific movements with his hips, knees, and ankles. These maps would then be encoded into the cranial implants.
Post-calibration, the brain-spinal interface immediately took effect. When the interface was enabled, the patient immediately regained muscle movement in his lower limbs with 97% accuracy.
He then naturally walked with the assistance of crutches, able to decide the initial step, continuous walking, and stopping with complete accuracy. As soon as the interface was disabled, natural function stalled, though neural signals continued to be detected.
After further calibration of the interface, the patient could also climb steep ramps, navigate high steps, and ascend stairs.
The brain-spinal interface also improved the patient’s neurological rehabilitation. Sensory and motor scores improved significantly, and conventional assessments such as six-minute walk tests, weight-bearing capacity, and others improved throughout the patient’s 40 sessions.
While the NeuroX Institute’s brain-machine interface is a triumph, there are some issues to note. Firstly, the system requires an external processor to transmit signals in real time. The processing unit is carried as a backpack or installed in a walker, meaning movement without these externals would not be possible at the current stage. Secondly, the conducted study only observed a single individual and further studies would be optimal before full release to the public. Thirdly, this system will be expensive, ruling it out for many.
Despite these drawbacks, however, the brain-spinal interface is a significant step forward in spinal injury motor restoration, opening the door for further advances in the years to come.
To read more of this series, please visit www.williamhaseltine.com

