Cancer Treatment CAR T Therapy Prolongs Survival Over Standard Care
(Posted on Sunday, June 25, 2023)
Originally published on Forbes on June 20th, 2023.

CAR T product Yescarta prolongs survival longer than standard care for patients with early relapsed or refractory large diffuse B cell lymphoma, according to new study results. This is an encouraging sign that CAR T therapy may continue to jump to earlier lines of treatment.
GETTY
Newly released study results demonstrate that Yescarta, a CAR T therapy, prolongs survival longer than standard care for patients with early relapsed or refractory large diffuse B cell lymphoma.
About the CAR T Therapy
Chimeric Antigen Receptor T therapy is a cell based treatment which takes a patient’s immune cells, enhances them, and reinfuses them to fight cancer. In 2017, biopharm company Gilead released their CAR T product Yescarta to treat adult patients with diffuse large B cell lymphoma (DLBL), an aggressive blood cancer which impacts 5.5 out of 100,000 Americans each year. The therapy is designed to attack cancer cells that carry a particular tag—antigen CD19—on their cell surface. Unique to this company, each CAR T cell possesses a CD28 costimulatory molecule to help the cell activate and release the proper signals to kill.
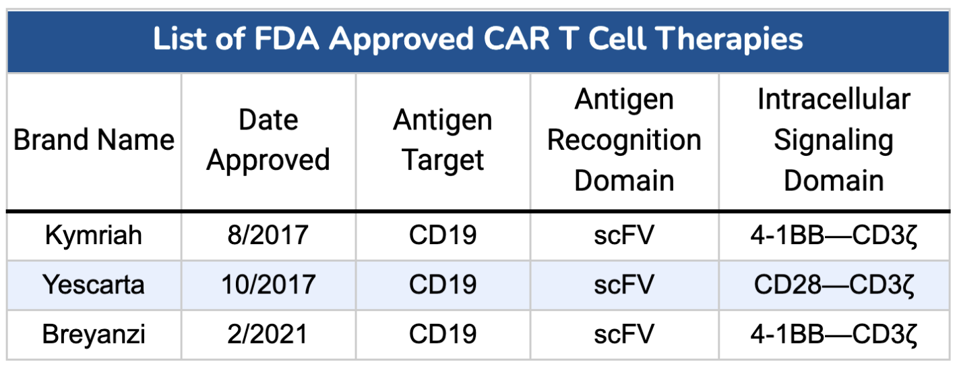
TABLE 1: List of CAR T products for treatment of diffuse large B cell lymphoma. All options target antigen CD19 using a single chain variable fragment (scFV) in the extracellular region of the receptor; however, only Yescarta wields a CD28 costimulatory molecule in the intracellular region.
ACCESS HEALTH INTERNATIONAL
It is difficult for lymphoma patients to qualify for standard treatments such as chemotherapy and stem cell transplants, and only around 20% who do are ultimately cured. CAR T therapy has posed as a viable option once other options run dry. To qualify, a patient initially needed to try and fail two or more previous lines of treatment. Due to later clinical trial success, the therapy is now approved for patients to use after their first treatment fails to achieve lasting results.
Despite the product’s success, an important question remained: how does this therapy, when administered early in treatment, perform over time? Gilead’s recent five year follow up study suggests that CAR T therapy can deliver encouraging results years after first injection—even outperforming standard care in certain arenas.
Study Design
In their study, Westin et al. recruited and randomized treatment for over 350 patients with diffuse large B cell lymphoma. To qualify, the participants either did not respond to first line treatment or had relapsed within 12 months after first-line chemotherapy.
Half of the patients received CAR T therapy, while the other half received standard care. Standard care entailed two to three cycles of platinum-based chemoimmunotherapy, followed by a combination of high dose chemotherapy and stem cell transplantation for patients who had partial to undetectable cancer. Treatment evaluation occurred five years after the first participant was randomly assigned to a treatment group.
Additional treatments were given in certain circumstances. Some patients received glucocorticoids to quell their cancer before receiving their CAR T cell injection. Similarly, some standard care participants received CAR T therapy due to disease progression or lack of response.
Result: CAR T Therapy Prolongs Survival
The CAR T therapy and standard care groups demonstrated significant differences in efficacy and patient survival. The results suggest that CAR T therapy prolongs survival better than standard care.
For example, around 55% of CAR T patients survived four years after first injection compared to 46% in the standard care group. Another telling marker included median overall survival, the amount of time after which half the patients have survived and half have died. The team could not calculate a median overall survival rate for the CAR T therapy group, as more than half of the patient pool did not die. In contrast, the median overall survival for the standard care group was 31.5 months.
What about potential complications? Here, too, CAR T therapy elicited better results. Four years down the line, around 42% of the CAR T group is expected to survive without their cancer worsening; this number lies near 24% for the standard care group. Half of the CAR T patients went an average of 10.8 months without their cancer returning or worsening versus 2.3 months for their study counterparts.
Crossover Participants
Interestingly, CAR T therapy illustrated a greater overall survival than standard care for “crossover” participants, referencing the 79 standard care participants who received a CAR T cell injection after chemotherapy. For these people, their cancer either worsened or did not respond at all to the chemotherapy and/or stem cell transplantation administered for the study.
Adverse Events
The safety profile for CAR T therapy aligned with previous results. Adverse events occurred for all participants, treatment group or otherwise. Notably, 74 patients from the CAR T group and 91 patients in the standard care group died during the study.
CAR T cell patients experienced expected adverse events for therapy. The majority suffered from mild to moderate cytokine release syndrome (CRS), a condition caused when the therapy aggressively activates the immune system. This group also reported higher rates for neurological events than the standard care patients; this included symptoms such as a generally confused state, loss of speech, and impacted brain function.
Future Implications
What is considered “standard” care for diffuse large B cell lymphoma may soon need reevaluation. Six years ago, patients with difficult to treat blood cancers underwent several standard treatments before turning to CAR T products. However, this study and others of its kind demonstrate that CAR T therapy may perform better than standard care even when administered early in treatment. Whether used alone or shortly after a previous treatment falters, this cell therapy may grant lymphoma patients a greater chance at surviving and potentially curing their cancer.

