Exploring The Potential Of Microrobots For Targeted Drug Delivery
(Posted on Wednesday, June 21, 2023)
Originally published on Forbes on 6/21/2023.
The following is part of a series on brain-machine integration and biomechanical solutions to restore function to tissues damaged by disease, trauma, or time.
Enhanced microrobots in our bloodstream may be the next step forward for therapeutic delivery technology. When a patient is administered a drug therapy intravenously, the drug is shot into the circulatory system and passively carried throughout the body by the natural pumping of blood. These remedies are among the most efficient, as oral medication can be impacted by the digestion process. In most cases, this is sufficient, particularly when an ailment is affecting a major organ such as the brain, heart, or lungs, for example.
Some tissues, however, are harder to reach. The eyes and gastrointestinal tract are among those difficult to treat via circulatory medicine. Blood flow in these areas is particularly intense, creating a challenging environment for medicine to passively disperse.
In response, Li et al. investigated using microrobots with claw attachments to aid medicinal delivery. These microrobots navigate the complex circulatory tract in harder-to-reach tissues, deliver treatment, then dissolve over time. Here we discuss their microrobots, both how they work and their potential impact on future treatment administration.
Microrobotics is far from a new field of study, with the earliest designs dating back to declassified military applications in the mid-1970s for reconnaissance and prisoner-of-war rescue. In more recent years, their medical applications have flourished, typically as delivery systems or diagnostics.
Though microrobots faced the same issues as drugs when faced with high-intensity blood flow regions of the body—swept away before they could deliver their cargo. Li et al. addressed this issue by observing microscopic aquatic life. The limnoterrestrial tardigrade, colloquially known as water bears or moss piglets, is among the most resilient animals on Earth, surviving in some of the planet’s toughest conditions, including high-speed waters. Using their claws, the creature grapples surrounding surfaces when the current grew too fast. This survival tactic was reengineered for the medical microrobots.

FIGURE 1: The microscopic tardigrade, approximately the size of a grain of sand.
Mimicking the claw-ground engagement mechanism of the Targigrade, microrobots were outfitted with claws of similar size, capable of burrowing into the membrane of neighboring red blood cells. The microrobots could be externally navigated via computer, and the claws retractable via a magnetic control system, both operated by a medical practitioner.
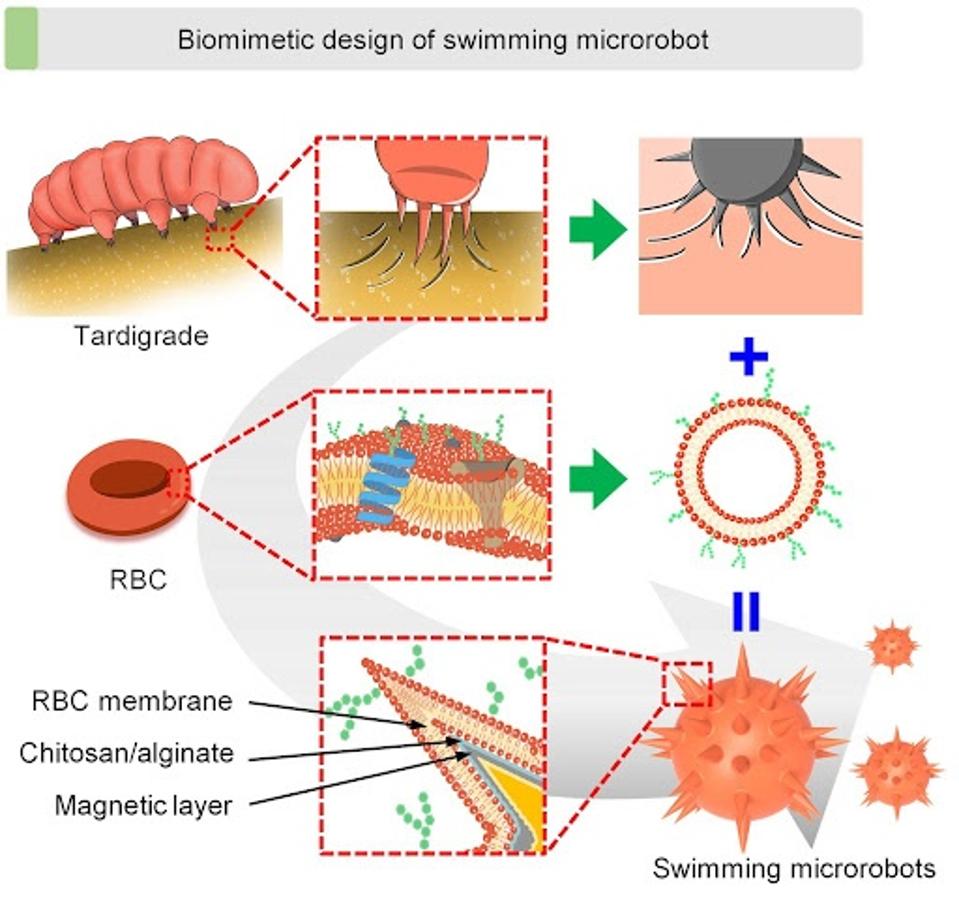
FIGURE 2: Conceptional development of claw-engaged and biolubricated swimming microrobots (CBSMRs). The tardigrades inspired the clawed geometry design and magnetically actuated claw engagement of the swimming microrobot, and the red blood cell (RBC) membrane–camouflaged coating was functionalized on the swimming microrobots to reduce the impact of the blood flow.
Comparing the clawed microrobots to control microrobots of similar size without claw mechanisms, Li et al. found that the clawed robots could withstand a blood flow sevenfold the force of those without claws before failure. They also found that the optimum size for clawed medical microrobots while accounting for blood flow force was about twenty micrometers, slightly larger than typical blood microrobots.
In live animal experiments, the microrobots safely and effectively navigated the veins of both mice and rabbits, maintaining resistance to blood flow and retaining in the blood with dissolving for sufficient periods.
Despite its early design and further research to be conducted, medical microrobots will be implemented into regular medicinal delivery practices. Their small size will allow for a relatively inexpensive production, the controllability factor will ensure the proper delivery of medication, and the enhanced claw technology will be a major step forward for hard-to-reach tissue remedies.
Further applications may include more advanced treatments, perhaps for cancer or circulatory diseases, and surgery assistance. We emphasize further research in this field prior to widespread use, but early indications are positive.

