The Future Of Cancer Treatment? Treating Multiple Myeloma With MRNA-CAR T Technology
(Posted on Monday, July 3, 2023)
Originally published on Forbes on June 27th, 2023.
At the frontier of cancer treatment development, there is hope for two modern technologies to combine and achieve unprecedented results: messenger RNA and Chimeric Antigen Receptor T Cell Therapy. Biopharm company Cartesian Therapeutics is determined to use this unique platform to benefit patients with a particular blood cancer called multiple myeloma. The company released preclinical trial results in the journal Nature Leukemia, and has a clinical trial underway. Here, we will explore the appeal behind joint mRNA-CAR T therapy for cancer and describe key findings from the preclinical trial.
Multiple Myeloma and CAR T Therapy
Multiple myeloma is a cancer caused by the irregular growth of a white blood cell called plasma cells. These cells, found primarily in the bone marrow, typically protect the body from infections by producing antibodies. However, when these cells turn cancerous, they interfere with other essential cells in the blood and bones.
Chemotherapy, targeted therapy and stem cell transplantation are three common treatment options for myeloma patients. In recent years, CAR T therapy has joined the ranks, albeit as a later stage alternative. Patients who turn to CAR T therapy after trying and failing five or more previous cancer treatments may finally see a significant, and sometimes complete, reduction in cancer signs.
How CAR T Therapy Works
CAR T therapy works by extracting and permanently strengthening a pool of immune cells—killer T cells—to eliminate a patient’s cancer upon reinfusion.
In the lab, the cells are bioengineered with DNA that encodes a new receptor. The antibody-like structure on the receptor called a single variable chain fragment (scFv) gives the T cell a heightened ability to detect cancer cells. The fragment attaches to B cell maturation antigen, or BCMA, on the cancer cell before triggering a signal cascade in the T cell. The T cell can then release its usual bevy of chemicals to destroy the cancerous target.
Shortcomings of CAR T Therapy
Although CAR T therapy can benefit patients with difficult-to-treat myeloma, there lies a major drawback: its toxic effects.
The therapy is unrelentingly rigid. The engineered cells are permanently set to detect and destroy myeloma cells. The covert disadvantage here is that the cells cannot be stopped if they go too far. Overstimulating the immune system like this can lead to potentially fatal side effects such as cytokine release syndrome (CRS) or neurotoxicity. It would be ideal to slowly stimulate the immune system, instead.
Adverse events can occur prior to the CAR T infusion, as well. Patients undergo a preparatory course of chemotherapy to eliminate some native immune cells. This practice makes space for the T cell infusion to proliferate once infused. As chemotherapy is notoriously hard on the body, it would be helpful to bypass this step, if possible.
Benefits of mRNA Technology
Enter mRNA technology. This innovation could single-handedly transform CAR T therapy into a safer and more flexible cancer treatment. The crux here is to temporarily instruct a patient’s T cells to fight cancer. The treatment can therefore be controlled in a repeated, dose-dependent manner. If less CAR T cells are infused at once, preparatory chemotherapy might also be avoided altogether.
Traditional CAR T therapy changes a T cell’s DNA. The new genetic information is folded into the genome and kept intact even as the cell replicates and multiplies. In contrast, messenger RNA is inherently unstable genetic information. As the mRNA inevitably degrades, receptor expression should likewise recede.
Optimistic Study Results
How feasible is this concept? Researchers Lin et. al tested this in their preclinical study using mice models of multiple myeloma.
Mice with humanized models of myeloma received an injection of humanized T cells each week for four weeks. The mice were then split into three groups, receiving either: 1) non-active substance (vehicle) to control for potential injection effects, 2) a control of killer T cells, and 3) Descartes-08, a CAR T therapy which modifies T cells to express anti-myeloma chimeric receptors for only a week. The mRNA encoded a single chain variable fragment (scFV); a CD28 molecule to support cell signaling; and a CD3 signaling domain responsible for killer signal cascade.
The control T cell and CAR T cell infusions were administered once a week after Day 7. Both the CAR T product and the control T cells relied on CRISPR gene editing to decrease expression of native T cell receptors. This prevents the intervention from being rejected by the host. Additionally, all of the mice were preconditioned with a chemotherapy drug called cyclophosphamide.
Of the three groups, the CAR T cell mice demonstrated the most prolonged survival; the median survival was significantly higher at 69 days. Comparatively, the vehicle-treated and killer T cell control mice began to lose weight and show signs of myeloma; the median survival lingered at 43 and 44 days respectively. This suggests that the transient CAR T therapy, when administered intermittently, can successfully control tumor growth and extend survival.
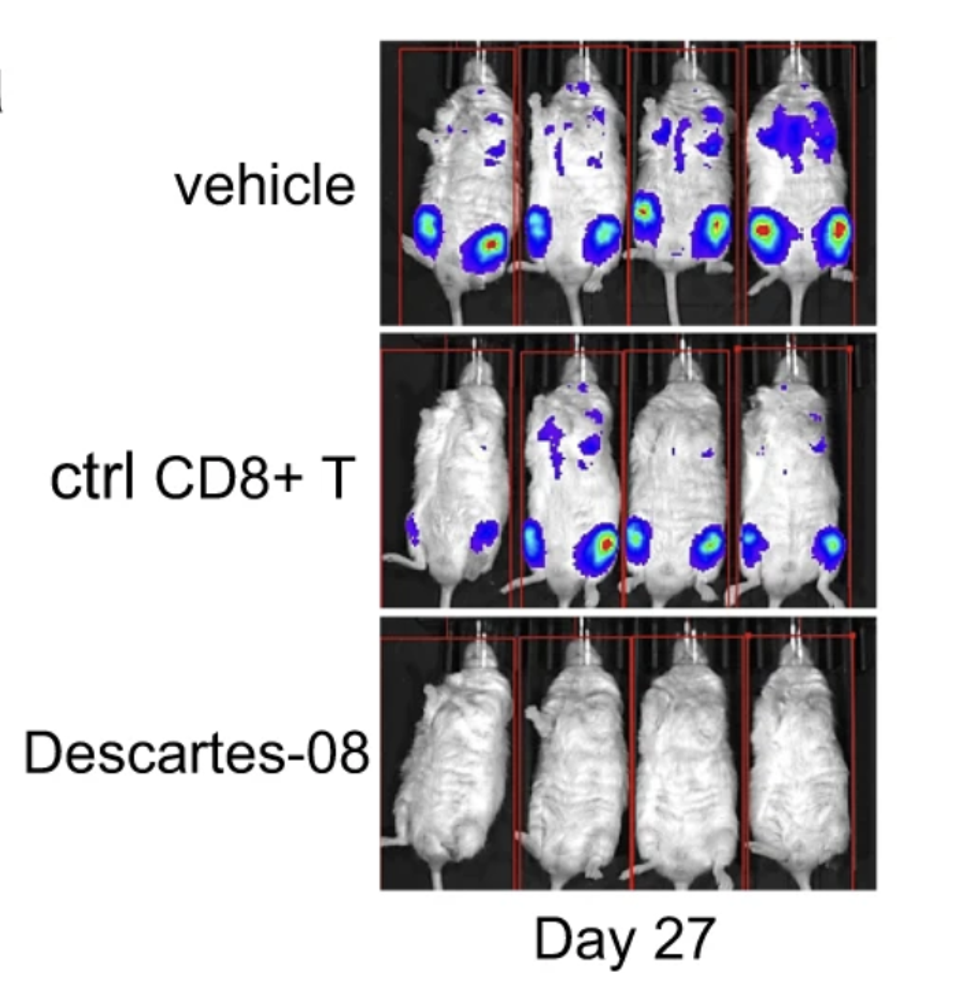
Figure 1: Comparison of tumor growth in mice models of multiple myeloma. As expected, the vehicle mice and mice who received a control of killer T cells (ctrl CD8+T) exhibited significant tumor burden at Day 27. The CAR T mice, in contrast, had yet to show signs of tumor burden.
LIN, L., CHO, SF., XING, L. ET AL. PRECLINICAL EVALUATION OF CD8+ ANTI-BCMA MRNA CAR T CELLS FOR TREATMENT OF MULTIPLE MYELOMA. LEUKEMIA 35, 752–763 (2021). HTTPS://DOI.ORG/10.1038/S41375-020-0951-5
Patient Example
The transient CAR T therapy also improved tumor burden for a 76 year old patient suffering from plasma cell leukemia.
Similarly to multiple myeloma, plasma cell leukemia involves the uncontrollable growth of plasma cells. Both illnesses share the same therapeutic target—B cell maturation antigen on plasma cells.
First, the patient received preparatory chemotherapy. This was followed by three CAR T cell infusions given over the span of two weeks.
The patient did not experience any adverse effects from the infusions. After twelve weeks, the patient had no residual signs of cancer. This is an encouraging example of what transient CAR T cells can accomplish for cancer patients. The next step is to test with a larger study population.
News on the Clinical Trial
A clinical trial is currently in progress. The study will see how advanced myeloma patients fare when given increasing doses of the aforementioned CAR T product. In Phase I of the study, none of the myeloma patients experienced cytokine release syndrome or neurotoxicity. This suggests that the treatment was well-tolerated and could potentially circumvent unwanted effects. The estimated trial completion date is set for next year.
Looking to the Future
Standard CAR T cell therapy, while effective, invokes its own risks. Integrating mRNA technology could be the solution to these prevailing issues. By transiently expressing the desired chimeric receptor, the therapy could be simplified and the safety could be improved all at once. Given the promising preclinical trial and case study results, we highly anticipate the clinical trial completion date.

