Breakthrough in Gene Therapy: Epidermolysis Bullosa
(Posted on Wednesday, August 23, 2023)
Originally published on Forbes on 8/17/2023

This story is part of a series on the current progression in Regenerative Medicine. This piece is part of a series dedicated to the eye.
In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
In a groundbreaking development, gene therapy delivered through eyedrops has restored the sight of Antonio Vento Carvajal, a teenager who has been legally blind for most of his life. Antonio was born with dystrophic epidermolysis bullosa, a rare genetic condition that causes blisters all over his body and in his eyes. After participating in a clinical trial for topical gene therapy, which initially targeted his skin, Dr. Alfonso Sabater had the idea to adapt the treatment for Antonio’s eyes.
The Vyjuvek, formerly known as B-VEC, treatment uses an inactivated herpes simplex virus to deliver working copies of the gene responsible for Antonio’s condition. The results have been remarkable, with Antonio’s vision significantly improving and the scarring in his eyes disappearing. This breakthrough offers hope for Antonio and opens the door to similar therapies that could benefit millions of people with other eye diseases.
Understanding Epidermolysis Bullosa
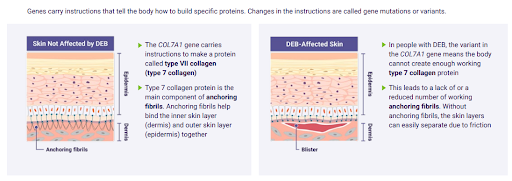
Epidermolysis Bullosa, commonly called EB, is a group of rare inherited skin disorders that cause the skin to be very fragile. A faulty gene mutation in COL7A1 causes the condition. Those with EB find that any trauma or friction to the skin can cause painful blisters to form.
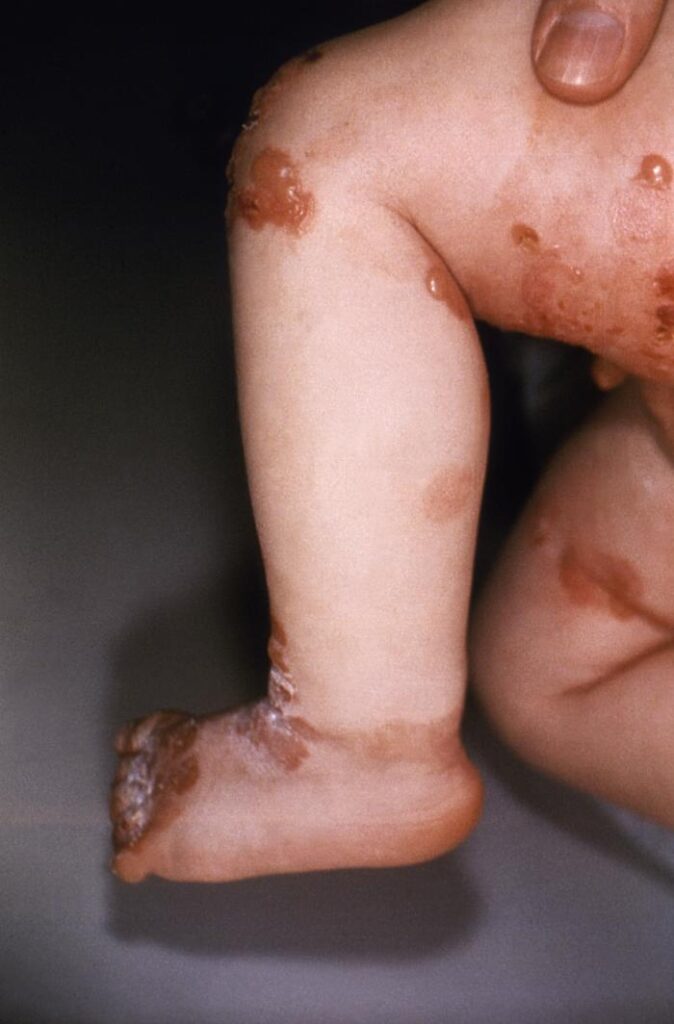
The severity varies from person to person and can range from mild to severe. There are also specific subtypes of the disorder that are host to different characteristics. Depending on the type and severity of EB, patients may also face complications such as infection, anemia, growth issues, and vision problems.
Most people with the disease are battling blisters across their hands and feet and thickened skin. As the skin constantly fights trauma, it will thicken over time, scar, and change color. Blisters can also form on the internal epidermis, such as in the mouth and through, making eating difficult.
Treatment for EB aims to relieve symptoms and prevent complications. Currently, it has no cure, so treatment focuses on supportive care. This care includes wound care, pain management, nutritional support, physical therapy, and psychological support.
One FDA-approved treatment for EB is currently available and making waves. This treatment is Vyjuvek or beremagene geperavec. It is a treatment specifically created for patients with dystrophic epidermolysis bullosa (DEB) and the first topical gene therapy to receive approval in the US.
It’s important to note that Vyjuvek is only approved for treating wounds related to dystrophic epidermolysis bullosa and not as a complete treatment for all types of epidermolysis bullosa.
How Vyjuvek Works
Vyjuvek is a gene therapy gel that delivers a healthy copy of the gene, encoding the protein type VII collagen to targeted skin cells. The protein anchors the layers of skin together to promote healing.
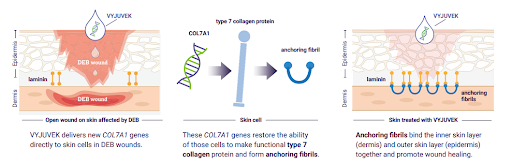
The drug delivers functional copies of the COL7A1 gene directly to the wound. It does this by using a modified herpes simplex virus type. The functional gene then produces a kind of collagen to start wound healing.
Before being approved by the FDA, Vyjuvek underwent two significant clinical trials. These trials aimed to assess the drug’s safety and effectiveness in treating wounds caused by DEB. Seventeen patients participated in the studies, and the medication was well-tolerated, with no severe complications reported. A phase III trial was also completed. After three months of treatment, significantly more wounds treated with Vyjuvek had healed than those treated with a placebo. After six months, 65% of wounds were healed in those using Vyjuvek compared to 26% in those using the placebo.
Given its positive safety record and promising trial outcomes, exploring alternative medicinal uses that could improve the quality of life for individuals affected by DEB seemed reasonable. The questions remaining are how did they do it and how did it restore a young boy’s vision?
Using Vyjuvek for Restoring Vision
Initially, the Vyjuvek formulation utilized a gel infused with a herpes simplex virus type to deliver the COL7A1 gene to wounds. However, further modifications were necessary to accommodate its use in the eye.
The new version of the formula does not contain gel, but the original formulation remains unchanged. This latest version allows Vyjuvek to be given through eye drops, which deliver the COL7A1 gene directly to the eye. This process efficiently generates collagen 7 within the cornea, crucial for maintaining its structural integrity. Insufficient levels of collagen 7 can cause corneal dystrophy, leading to vision impairment. However, with Vyjuvek, it is now possible to restore collagen 7 levels and reverse the effects of corneal dystrophy, ultimately resulting in improved vision.
This method has seen success in the recent trial with young Antonio. Still, it was only one case that was under “compassionate use” approval from the US Food and Drug Administration. Those involved in Antonio’s case exercised extreme caution and were always considering the safety of the treatment. This caution was significant as Vyjuvek doesn’t modify DNA, so it’s not a one-time treatment like many gene therapies.
Ultimately, the trial was successful as Antonio’s eye fully recovered from the surgery, and there has been continuous improvement every month without any scarring. The results were so promising that the medication is now allowed off-label use for the eyes.
Though there may not be specific plans for clinical trials of Vyjuvek for eye diseases at the moment, the success of this therapy in restoring vision in dystrophic epidermolysis bullosa patients opens up possibilities for future research and development in the field of gene therapy for other eye diseases.
To learn more about the eye, read more stories at www.williamhaseltine.com

