Unknown Player, Untapped Potential? Using Gamma-Delta T Cells For CAR T Therapy
(Posted on Saturday, September 23, 2023)
Originally published on Forbes Sep 18, 2023.
A lesser-known population of T cells has the potential to improve several limitations of
GETTY
In the ever-evolving world of cell therapy, researchers often turn to T cells—a type of white blood cell—to transform the way we treat cancer. This is true for CAR T therapy, a modern cancer intervention that borrows and boosts the power of killer T cells to counter certain lymphomas, leukemias and multiple myeloma.
Within the T cell family lies a player of unknown and untapped potential, a subset known as γδ T cells. Though their role in the immune system remains largely veiled, γδ T cells are gaining popularity as a possible means to improve current immunotherapies.
This article will explore the function of γδ T cells in the immune system, their potential to improve cell therapies such as CAR T therapy, and current setbacks to adopting these cells on a clinical level.
All About γδ T cells
To understand γδ T cells, it is first important to understand the general role of T cells in the immune system.
T cells are critical components of the adaptive immune system, the body’s slower but more tailored response to foreign threats. T cells usually fall into one of two categories: CD4+ “helper” T cells or CD8+ “killer” T cells. These T cells concert with other immune cells, recognizing and reacting to specific signs of invasion.
T cell receptors (TCRs) facilitate this recognition through a two-step activation process. An antigen-presenting cell, such as a dendritic cell or macrophage, will first show the T cell receptor what to look for: a target antigen peptide, or more simply, a protein tag from the invader. The peptide is displayed on a cell surface molecule called the major histocompatibility complex (MHC). The T cell receptor binds to the antigen-MHC complex, initiating the first activation signal. A second costimulatory signal, typically provided by costimulatory molecules on the antigen-presenting cell, is received to complete T cell activation. Once activated, the T cell can correctly identify the antigen-MHC complex on other cells in the body—including on infected or cancerous cell targets.

FIGURE 1: Most T cell receptors (TCR) are composed of an alpha and beta protein chain. This region binds to peptide-major histocompatibility complexes (MHC) on other cells. CD8+ “killer” T cells bind to antigens presented by Class I MHC molecules, while CD4” “helper” T cells bind to Class II MHC molecules.
ACCESS HEALTH INTERNATIONAL
Small Structural Difference, Big Impact
The vast majority of T cells equip a T cell receptor composed of alpha and beta protein chains. Most research on T cells refers to these αβ TCR-wielding cells. However, there exists a minority population of T cells that carry T cell receptors made of gamma and delta chains instead. Recent research suggests that these cells grow in a three-step process within the thymus. After this education, they reside in various tissues and mucosal surfaces, including the skin, intestines, lungs, uterus and lymph nodes.
This one structural difference may lend γδ T cells an entirely distinct skill set from their conventional counterparts.
For example, research shows that γδ T cells do not require prior exposure to antigens as typical T cells do. In fact, these cells are capable of binding directly to target antigens and other nonpeptidic antigen structures. γδ T cells can therefore form a more rapid and generalized cytotoxic immune response. Even though T cells usually form adaptive immune responses, this type of rapid recognition is more characteristic of innate immune cells, which provide the body’s initial, broad defense against pathogens and abnormal host cells.
In a similar vein, γδ T cells are known to recognize a wide variety of antigens. They can interact with stress-induced molecules on the surface of stressed and damaged cells. Their functions may vary depending on their location, as γδ T cells—though small in number—appear to be diverse and may have specialized functions attuned to specific tissues or microenvironments. These functions include defending against infections, repairing wounds and influencing other immune cells through the release of chemicals called cytokines.
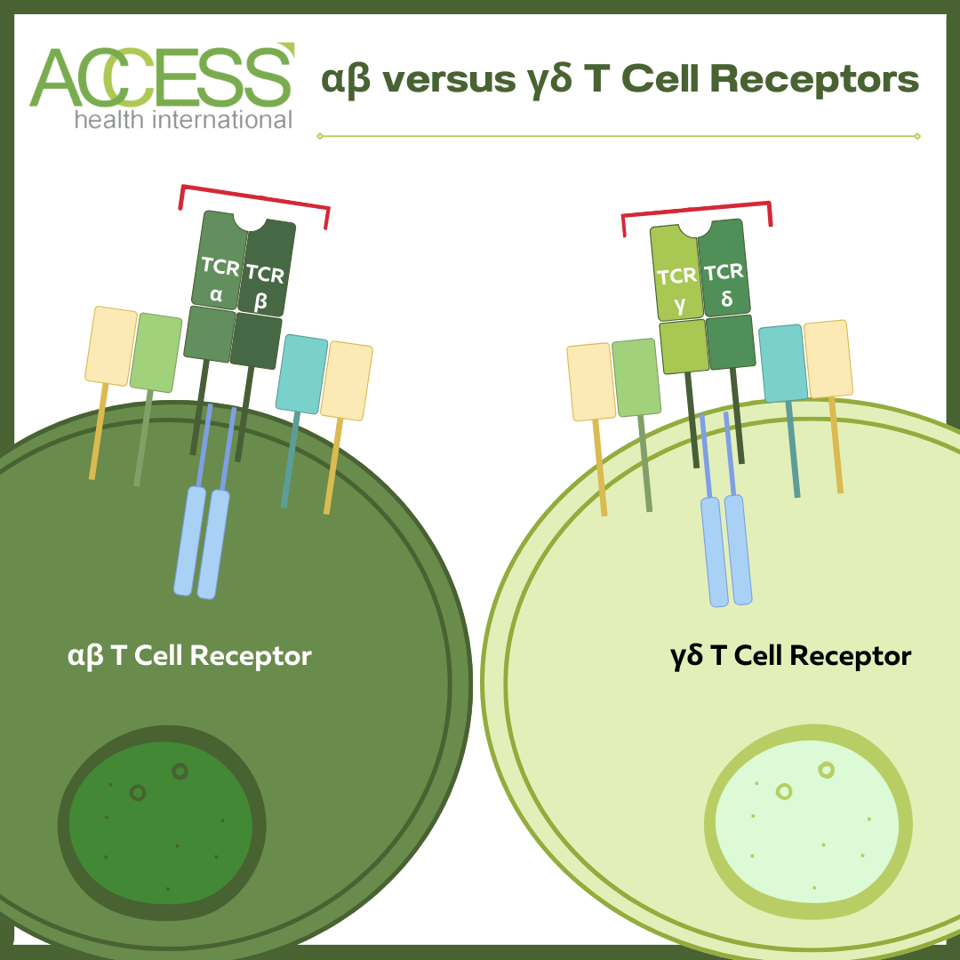
Structure comparison between αβ and γδ T cell receptors (TCRs). αβ T cells constitute the majority of T cell leukocytes.
ACCESS HEALTH INTERNATIONAL
The Appeal for CAR Therapy
CAR T therapy traditionally relies on CD8+ “killer” T cells that sport αβ T cell receptors. Why, then, would researchers be interested in making a cell therapy out of a small population of cells such as γδ T cells?
γδ T cells possess several attractive traits for cell therapy. For one, γδ T cells demonstrate potent anti-tumor responses for both blood and solid cancers. Their binding interactions are not limited by the presence of MHC molecules, which adds to their increased scope of recognition. Additionally, researchers hope to activate these locally based cells to attack nearby solid tumors.
γδ T cells may also be useful in combating the loss of antigen targets associated with traditional CAR T therapy. Even when chimeric antigen receptors (CAR) are attached to their cell surface, γδ T cells retain their ability to interact independently of major histocompatibility complex molecules; they can continue to target cancer cells even if they begin to downregulate the target antigen.
The ability to function across MHC barriers also means that a CAR T infusion made from donor cells is less likely to be rejected by the body. This works because major histocompatibility complexes, which are present in almost all cells with a nucleus, help the body distinguish between itself and enemies. Rejection, otherwise known as graft-versus-host-disease (GvHD), occurs when the body does not recognize the foreign MHC molecules on the donated cells. The body then attacks the graft as if it were a threat. Current off-the-shelf investigations using αβ killer cells suffer an increased risk of rejection for this reason.
Finally, γδ T cells are less prone to hyperactivation, decreasing the risk of adverse effects such as cytokine release syndrome and T cell exhaustion as a result.
Caveats to Clinical Translation
Research on γδ T cells is optimistic but largely preliminary. γδ T cells are not conserved between mice and humans, making comparisons difficult. This means that the long-term effects of this therapy on humans are mostly unknown. Clinical trials against solid tumors have yielded limited success thus far.
Looking Forward
Taking advantage of CAR T therapy’s flexible platform, it could be beneficial to attach chimeric receptors onto γδ T cells instead of their more conventional αβ killer T cell counterparts. γδ T cells possess intrinsically favorable qualities for cancer treatment. Nonetheless, further experimentation is required to understand how to harness these qualities on a clinical level.


