Bridging Gaps for Affordable Cell and Gene Therapies: Overcoming Financial and Systematic Obstacles
(Posted on Wednesday, October 18, 2023)

This story is reprinted with the kind permission of Inside Precision Medicine.
Regenerative medicine has emerged as a promising treatment option for chronic medical conditions. Specifically, cell and gene therapies, innovative forms of regenerative medicine, have shown great potential in personalizing and targeting disease treatment. These cutting-edge therapies leverage a patient’s cells and genes to repair and regenerate damaged tissues and organs.
However, despite their potential benefits, the high cost of these therapies presents significant challenges to accessibility. This lack of accessibility underscores the urgent need for continued research and development to make these treatments more affordable and accessible to needy patients.
Understanding cell therapies
Stem cells are unique cells that have the potential to develop into a variety of different cell types in the body. They serve as the building blocks from which all other cells in the body are derived. These cells are characterized by their ability to regenerate themselves through cell division and differentiate into specialized cells, such as muscle, nerve, bone, and blood cells.
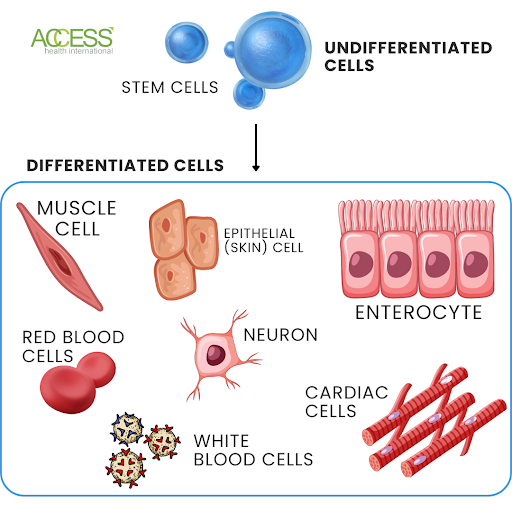
FIGURE 1: Infographic representation of undifferentiated cells and differentiated cells.
Embryonic stem cells were described in the 1950s, surpassing adult stem cells in their ability for self-organization, although stem cells were first recognized in the 19th century. This realization led to much research into their use in treating various medical conditions. In the 1970s and 1980s, more researchers became aware of the potential medical applications of stem cells. By 1998, James Thomson at the University of Wisconsin had developed a way to isolate and culture human embryonic stem cells from donated embryos.
Since then, there has been an explosion in research on stem cell technology and its potential applications. Today, researchers are progressing toward understanding how to control cell differentiation for use in tissue engineering and regenerative medicine. For example, using induced pluripotent stem cells generated from adult skin or blood samples can provide a highly flexible source of stem cells.
Furthermore, research has shown that stem cells may be capable of self-renewal, enabling their use in treatments for degenerative diseases such as Alzheimer’s, Parkinson’s, stroke, and spinal cord injury. In addition, some researchers believe that stem cell therapy may also effectively treat more chronic illnesses such as cancer or autoimmune diseases.
To use stem cells for medical purposes, they must first be isolated from their source – either the embryo or other tissue source – and then prepared in the laboratory by various techniques, such as sorting them according to their specific characteristics. After preparation, the stem cells can be injected into the patient’s body to transform into the required tissue for treatment.
What are gene therapies?
In the 1960s and 1970s, scientists began exploring ways of using altered DNA sequences within living organisms to promote healing inside damaged cells. This process is known as gene therapy. The National Institutes of Health conducted the initial human gene therapy trial in 1990.
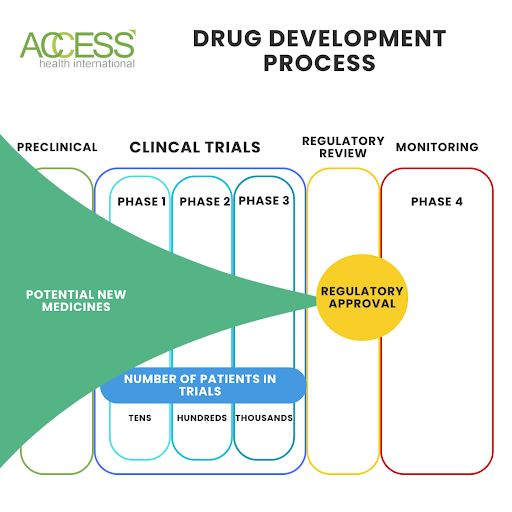
FIGURE 2: Infographic illustrating the drug development process.
The field of gene therapy has been a promising avenue for treating a variety of genetic conditions, including cystic fibrosis. This innovative approach involves replacing or repairing defective genes with functional ones, enabling normal cellular metabolism, facilitating healthy tissue growth, and supporting efficient repair processes. By addressing the condition’s root cause, gene therapy can provide long-term relief and improved quality of life for those impacted by genetic disorders.
Manipulating genes to repair or replace defective or missing parts of cells is known as “gene editing,” which inserts specific genetic material into human cells to modify their functionality and can take genetic material from healthy cells of patients or donors. The goal is to restore normalcy in defective genes responsible for causing disease.
The added genes may be supplied through DNA-carrying vectors like viruses, which act as delivery vehicles, carrying new genetic instructions into targeted tissues or organs. Once inside their target cells, these vectors release their contents, allowing them to take effect inside the body’s cells.
Three primary vector types are used in modern medical practice: viral vectors, non-viral vectors, and cell therapies. Each is unique in delivering therapeutic molecules into target cell sites where they are needed most. Once delivered, they produce therapeutic effects by manipulating the host genome at different levels, such as DNA level, RNA expression level, or protein level.
Viral vectors rely on virus particles as delivery vehicles carrying therapeutic molecules along with them until they reach target cell sites. Non-viral vectors do not use viruses but other methods such as liposomes, nanotechnology device systems, and scaffolds. Each has its advantages depending on the desired application of gene therapy.
Also, like any new medication, these treatments need to undergo rigorous testing and determine best practices for manufacturing before they reach patients.
Drug development, clinical trials, and the complexities of personalized medicines
During clinical trials, drugs go through three development phases. Phase I tests safety and side effects on a small group of healthy volunteers. In Phase II, a larger group with the targeted condition receives the medication to evaluate its efficacy and potential adverse effects. Phase III includes an even larger group in a randomized, double-blind, placebo-controlled trial to confirm earlier results and assess safety in a real-world setting.
After completing these trial phases, the drug undergoes a meticulous evaluation for regulatory approval. This comprehensive assessment encompasses the drug’s safety, efficacy, and manufacturing process. This evaluation process can take several years. Should the drug pass this stage, it may receive approval for use in the broader population.
The development process for specific medical treatments, such as gene or cell therapy, can be lengthy and costly. It can take up to 30 years to fully develop and test the treatment before it is available to patients. Additionally, the development process can cost billions of dollars due to the extensive research, clinical trials, and regulatory requirements to ensure the treatment is safe and effective.
Cell and gene therapy requires an even more complex process that uses specialized equipment and trained personnel. Ensuring sterile extraction and processing of cells and customized manufacturing for each patient can increase the cost of gene and cell therapies. However, despite these challenges, the gene and cell therapy markets are experiencing rapid growth.
Exponential growth for cutting-edge therapy development
According to estimates, the stem cell therapy market is worth over $11.8 billion. Meanwhile, the gene therapy market is expected to grow from $7.6 billion in 2022 to $44.5 billion in 2023.
Oncology has emerged as the leading therapeutic area in these novel treatment fields. This isn’t surprising, considering there were over 18.1 million cancer cases worldwide in 2020. The increase in the global disease burden of cancer has driven significant investments in novel approaches. Major government programs centered on cell therapies for cancer treatments encourage clinical and drug discovery focus on cancer treatments.
The Food and Drug Administration, FDA, has also implemented measures to expedite drug approval to make these treatments more feasible for development. One of these measures is the Breakthrough Therapy Program, introduced in 2012 and updated in 2018. With this program, the FDA has approved almost 140 breakthrough therapies, reducing the time it takes to approve new drugs by nearly a year.
Another similar measure is the introduction of a Regenerative Medicine Advanced Therapy designation (RMAT), which identifies investigational drugs that meet requirements for treating severe conditions with clinical evidence of addressing unmet medical needs. RMAT does not guarantee faster approval, but it is an expedited program similar to breakthrough therapy.
By 2025, the FDA expects to approve 10 to 20 cell and gene therapy products annually, many designed to be one-time curative treatments. Despite the evergrowing market and efforts by the FDA to streamline development processes, these life-saving treatments remain inaccessible to many due to their high price.
Outlandish prices for life-saving treatments
One therapy called Hemgenix® was approved as a one-time treatment for hemophilia B by the FDA. It comes with an estimated $3.5 million price tag. Another gene therapy for spinal muscular atrophy, by Novartis, called Zolgensma® , comes at a whopping $2.1 million.
Cell therapies also come with considerable price tags. One such is Kymriah®, used in lymphoma patients, which comes in at $280,000 for those in Germany and $475,000 for those in the U.S. Due to its high price tag, Irish payer systems have determined that Kymriah is not a cost-effective therapy compared to chemotherapy options.
Yescarta®, another cell therapy for cancer, has a similar list price of between $373,000 and $475,000. In the months immediately following the drug’s approval, only five people could afford the treatment due to high costs and payment challenges. The lag in determining a payment model with both government and commercial payers resulted in a waitlist of patients who could benefit from Yescarta but faced reimbursement or payment challenges that prevented them from receiving the needed treatment.
In 2018, The Centers for Medicare & Medicaid Services (CMS) decided it would follow the typical U.S. fee-for-service model and pay out $395,380 on an outpatient basis for Yescarta. This agreement comes with a published co-payment of $79,076 for patients, meaning that to receive the Yescarta treatment, they must pay almost $80,000 out of their pocket. That’s a massive amount compared to the average U.S. salary of $59,428.
So, while developing these treatments is critical, does it matter if the treatments are not accessible to those who need them most?
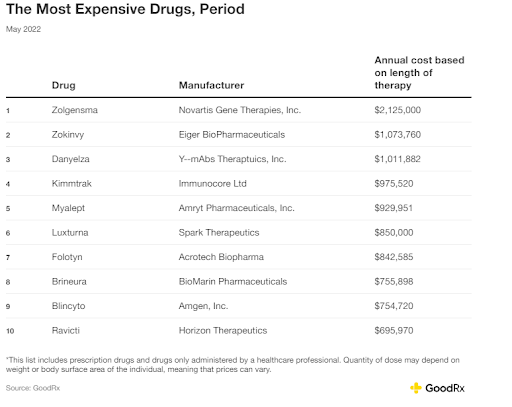
FIGURE 3: A GoodRx depiction of the most expensive drugs administered by healthcare professionals from 2022.
Paying for health in the United States
One of the biggest hurdles in making these therapies accessible is securing coverage and reimbursement for those substantial price tags.
In the United States, two primary payment systems are in place: private and public payers, which operate within a more extensive multi-payer system. Private payers are insurance companies that charge premiums for coverage. In contrast, public payers, such as Medicare and Medicaid, are funded by taxes and offer services at reduced or no cost for those who qualify.
The most prevalent setup in the U.S. healthcare system is the fee-for-service model, which presents certain limitations. Specifically, the per-dose pricing payment structure is unsuitable for cellular and gene therapies due to their significant upfront costs. Aside from these obstacles, there are also issues with the consistency in federal legal requirements for coverage related to cell and gene therapies.
Furthermore, most Medicaid programs rely on the diagnosis-related group (DRG) system to compensate hospitals for inpatient services. This system offers a bundled payment to the hospital for both the services provided and any drugs administered. However, the reimbursement structure may disincentivize hospitals from delivering cell and gene therapies to patients, as the costs may be considerably higher than the reimbursement amount.
In commercial coverage, plans are not federally required to cover specific drugs. However, individual and small group plans must cover essential health benefits (EHBs), which set minimum standards for prescription drug coverage. With the emergence of cell and gene therapies, programs and employers seek ways to provide access while managing high upfront costs.
Given the high cost of these treatments and coverage issues, there is concern that some patients may not have access, exacerbating health inequalities.
A method to combat cost
One proposed method to combat the complications of a traditional healthcare payment setup is implementing outcomes-based contracts. This payment setup means that manufacturers of treatments only receive reimbursement for treatment if the patient successfully reaches a predetermined clinical endpoint. This method gives healthcare payers peace of mind that they are not spending money on less effective therapy while allowing payer systems to share risk with manufacturers.
Interestingly, Yescarta adopted this approach in Europe and found success. Researchers also found that countries that were initially less open to outcomes-based reimbursements were becoming increasingly interested in this model due to the success seen by Yescarta in the United Kingdom and France. Other research published in Drug Discovery Today suggests a growing interest in this reimbursement model in central Europe and the Middle East. However, most countries in these regions do not currently use it.
This method aligns with the Institute for Clinical and Economic Review’s (ICER) views as their president, Steve Pearson, MD, said, “We have to be able to distinguish what’s really bringing value and what’s not” in a recent interview. He also highlights it being necessary to have positive clinical data to back up the significant price tags associated with these types of therapies, which is one of the primary pillars of this type of reimbursement program.
Outcomes-based approaches in practice
Spark Therapeutics uses a hybrid of outcomes-based and payment-in-installation models for their gene therapy called Luxturna® , which treats inherited retinal diseases. In their model, they must demonstrate Luxturna’s short-term efficacy within 30-90 days and long-term durability of 30 months to receive reimbursement. They also offer payers the option to pay in installments over several years.
Luxturna was the first direct gene therapy approved in the United States, and as such, it is setting the groundwork for policy and expectations of future gene therapies. In fact, Bluebird Bio recently adopted similar setups for their new treatments.
Bluebird Bio developed Zynteglo®, a treatment for transfusion-dependent beta-thalassemia. They are combining initial upfront payment with an outcomes-based fee schedule. They also take this setup a step further with an agreement to reimburse commercial and government payers 80% of the cost of therapy if a patient fails to achieve their clinical outcome benchmark.
GlaxoSmithKline, or GSK, was also setting up to take a similar payment approach before their decision to abandon their cell therapy products earlier this year. Instead, GSK has focused on oligonucleotides, short DNA and RNA molecules that regulate gene expression, and RNA-targeting modalities. However, these novel gene therapies still face the same costs as their predecessors, and it remains to be seen if the company will follow its cell therapy reimbursement plans for these new treatments.
The success seen with outcomes-based payment and reimbursement schedules comes right on the heels of an executive order from President Biden in October of 2022. The order, “Lowering Prescription Drug Costs for Americans,” directs the Department of Health and Human Services (HHS) to find ways to lower medication costs. The HHS has taken various steps to reduce costs and improve accessibility to care across the U.S. since the order.
Recent proposals that enable accessibility
In one of the many changes to meet the demands of the October order, the Centers for Medicare & Medicaid Services (CMS) may alter the rules for providing separate payments for cell and gene therapies. This change comes from a proposed rule published in May 2023. The proposed rule may require drug manufacturers to pay a rebate to the state if drugs are reimbursed as part of a bundled payment.
It also involves the implementation of a “drug price verification survey,” which aims to collect comprehensive information on the costs, use, and pricing strategies associated with high-priced cell and gene therapies. The gathered data will provide valuable insight into the pricing patterns of these therapies and enable a more informed decision-making process regarding their accessibility and affordability.
Another federal initiative announced earlier this year, the Cell and Gene Therapy Access Model, involves CMS coordinating and administering outcomes-based agreements with participating manufacturers for particular cell and gene therapies on behalf of state Medicaid agencies. These agreements could be outcomes-based payments, rebates, or annuities, with continued cost dependent on the benefit’s durability.
The Center for Medicare and Medicaid Innovation released a report in February 2023 outlining the models chosen for testing based on their affordability, accessibility, and feasibility of implementation. The report recommended a bundled payment approach for Medicare compared to an outcomes-based access model proposed for Medicaid, replacing fee-for-service billing during cell and gene therapy care episodes.
Where do we go from here?
Changes to coverage and plans to lower prescription prices will help alleviate the extreme financial burden that drives the inaccessibility of cell and gene therapies. However, there are additional factors that affect accessibility and require attention. These factors encompass the availability of treatments and the optimization of the supply chain.
If we address these factors and reduce out-of-pocket costs for patients, cell and gene therapies could become more accessible and common as life-changing treatment options. If these therapies develop a solid foundation, they will provide insights into the future of medicine and regenerative medicine.
Regenerative medicine has witnessed many groundbreaking innovations, with these therapies merely scratching the surface. Promising frontiers like biomaterials, organoids, gene therapies, brain-machine interfaces, and more are undergoing thorough investigation, presenting unprecedented prospects for novel treatments and transformative therapies.
By integrating these advancements with traditional and precision medicine, we can unlock new possibilities for improving health outcomes and extending the quality of life for millions of individuals worldwide.

