MicroMachines: Advances In Biorobotic Regenerative Medicine
(Posted on Friday, December 22, 2023)
This article was originally published on Forbes on 12/22/23.
This story is part of a series on the current progression in Regenerative Medicine. This piece discusses advances in robotics.
In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
How small is the smallest robot? Some might visualize a machine that could fit on the palm of your hand or the tip of your finger. In recent years, robots have been developed so small, they are barely perceptible to the naked eye, if at all.
Anthrobots, or biobots, as described by Dr. Gizen Gumuskaya and colleagues from Tufts University in a recent study, are spherical multicellular robots derived from our own human lung cells. The biobots range in diameter from only 30 to 500 micrometers. For context, the edge of a penny is over 1500 micrometers.
An exciting invention in their own right, but how can we use biobots in a regenerative medicine context? Gumuskaya and colleagues found that the autonomous robots could self-organized repair of tissue damage, in essence, patching up a scratch without human direction. This capability could open the door to a range of future applications.
Here, I will analyze these biobots and discuss their use in the future of medicine.
The biobots are developed by isolating human lung cells and manipulating them into spheroids. This is achieved by allowing the cells to soak in a liquid environment over time, enabling a uniform shape. These cells are covered in cilia; hairlike strands help lung cells clear debris and microbes from the lungs. In the spheroid form, the cell structure is uniformly surrounded by cilia. The cilia drive the motive capacity of the biobots, akin to legs rolling the cell along.
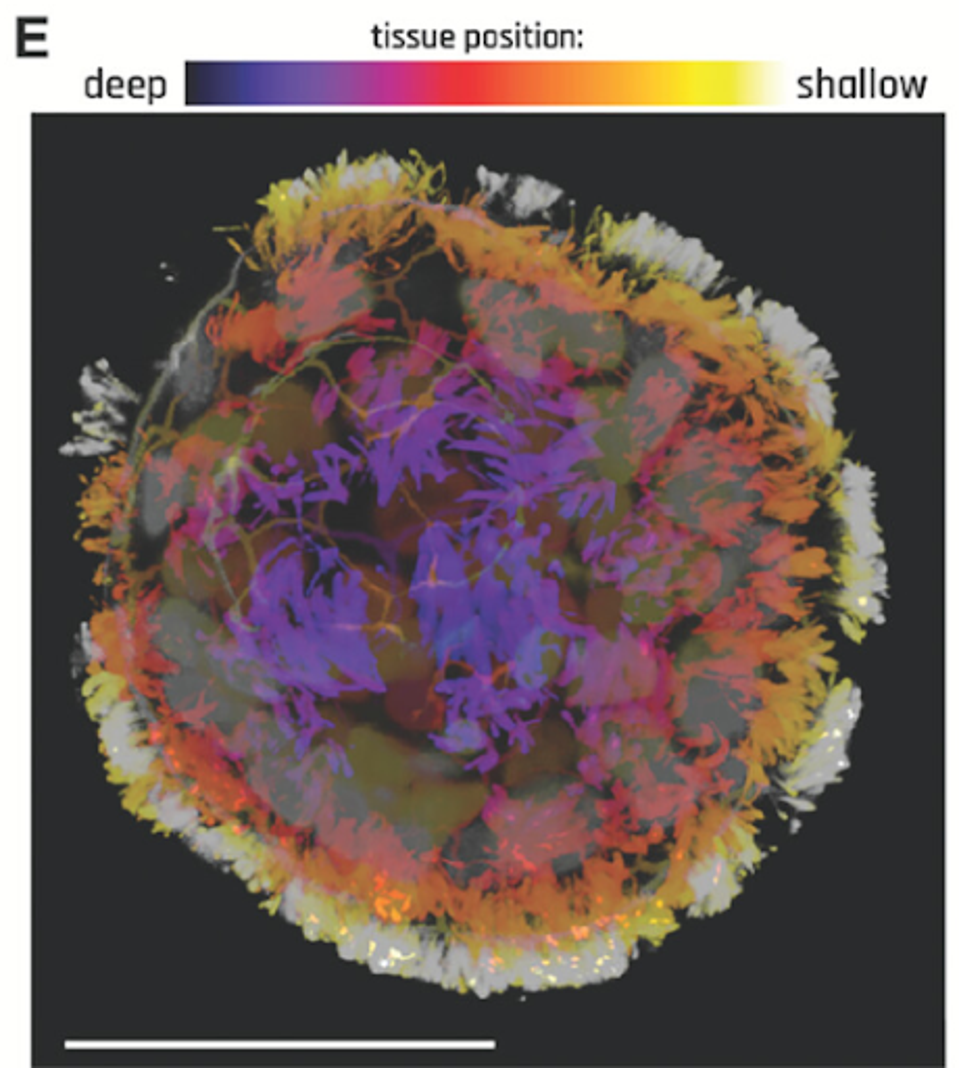
FIGURE 1: Anthrobot with depth information to show full cilia coverage.
The biobots most typically moved in patterns. These motility patterns ranged from tight loops to straight lines and speeds ranging from 5-50 microns/second. The researchers found that once a biobot has an established pattern of cilia movement, it is unlikely to switch to another type, suggesting a distinct motility fingerprint on a cell-by-cell basis.
While analyzing the interaction between biobots and environmental variability, they exposed the biobots to isolated somatic tissues, notable with damage sites. Lung cells do not typically interact with damaged tissues, particularly those not found within the lung.
Not only did the biobots traverse the simulated scratches on the tissue successfully, but they also found that biobots with higher rotational tendency or speed outperformed other biobots in coverage efficiency regarding tissue damage specifically.
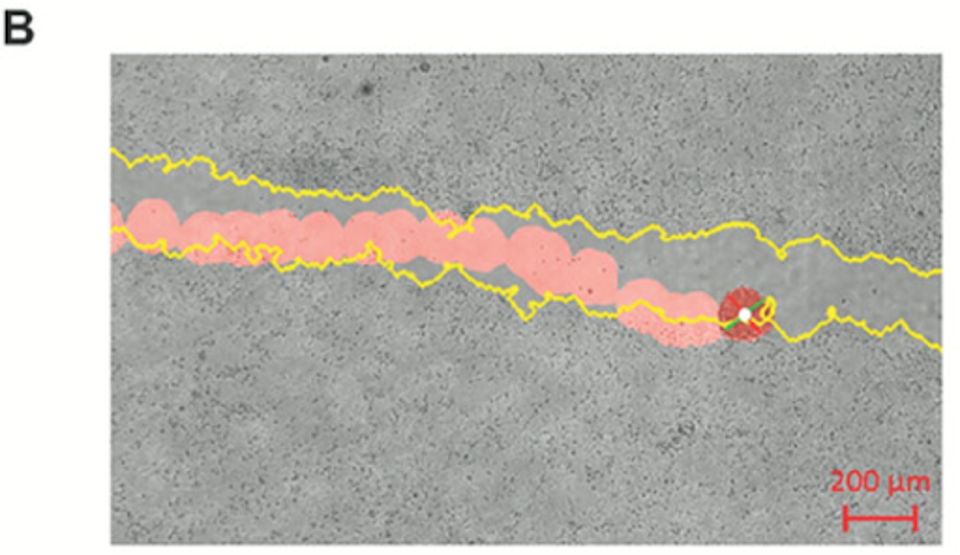
FIGURE 2: Sample tracking video output with scratch edge highlighted in yellow and bot path in red.
Perhaps most notably, the researchers found that biobot exposure to the scratch enabled cellular regrowth. Gumuskaya and colleagues enabled aggregation of the biobots to create a “superbot” that could bridge the gap of the scratch. Three days following the bridged gap, native tissue had grown in the site at a substantially faster rate. In a sense, the biobots stitched the site back together.
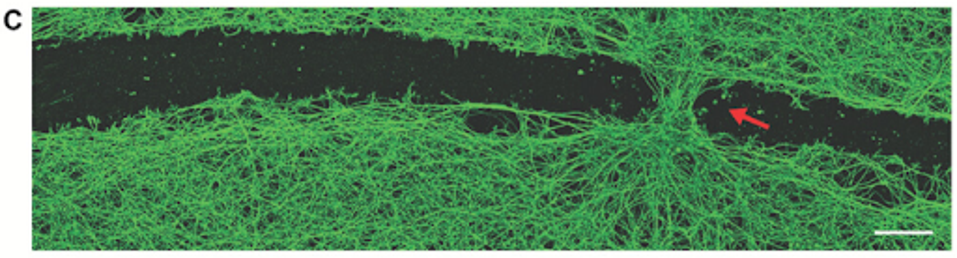
FIGURE 3: Induced neural gap closure at the site of bot settlement.
How can we apply biobots in an everyday medical context? The promise of autonomous mechanical stitches comes with lofty possibilities. Most directly, they can be used in a regenerative context to repair smaller wounds or drug delivery without needing medical technician assistance.
More abstractly, I could see a future where similar biobots are used to locate and eliminate harmful cells throughout the body, including potentially cancer cells.
Further application lies in the manipulation of somatic cells themselves. What else can we derive from our cellular potential if we can take a human lung cell and create a motile, autonomous repair robot?
Of course, these suggestions come with years of research, development, and testing, but as with many of the latest innovations in biomedicine, the future is arriving rapidly.

