A Promising New Approach For Glaucoma
(Posted on Tuesday, January 23, 2024)
This story is part of a series on the current progression in Regenerative Medicine. This piece is part of a series dedicated to the eye and improvements in restoring vision.
In 1999, I defined regenerative medicine as the collection of interventions that restore tissues and organs damaged by disease, injured by trauma, or worn by time to normal function. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
Glaucoma is a debilitating eye ailment that can cause irreversible harm to the optic nerve and eventually result in loss of vision. The condition is often asymptomatic in its early stages, making it challenging to diagnose and treat. It occurs when the fluid pressure inside the eye increases, damaging the optic nerve and causing vision loss. Fortunately, recent medical breakthroughs in RGC (retinal ganglion cell) replacement therapy offer hope for those suffering from this condition. This revolutionary treatment involves transplanting healthy RGCs to replace the damaged ones and restore visual function. Doing so aims to prevent further damage to the optic nerve and potentially reverse the damage already done. With this new treatment, there is hope for individuals with glaucoma.
What are Retinal Ganglion Cells?
Retinal Ganglion Cells (RGCs) are specialized neurons that play a critical role in the visual system by transmitting information from the retina to the brain via the optic nerve. These highly complex cells comprise more than a dozen molecularly, functionally, and topographically unique subtypes. Each subtype has distinct morphology, connectivity, and response properties, allowing it to perform different visual system functions.
RGCs are the final output neurons of the retina. They are responsible for encoding visual information into electrical signals sent to the brain. These signals are then processed by higher visual centers in the brain, which enables us to see and perceive the world around us.
Despite their complexity, RGCs have been a promising target for therapeutic intervention as they do not regenerate naturally once they deteriorate. Any damage to these cells can result in permanent vision loss. Scientists are actively working to develop new therapies that can help protect and restore RGCs in individuals with retinal degenerative diseases such as glaucoma and age-related macular degeneration.
Stem Cells & Organoids for Glaucoma
Stem cell-based transplantation has emerged as a viable option for replacing lost or damaged retinal ganglion cells (RGCs). As stem cells can differentiate into various cell types, including RGCs, they present a potential source for RGC replacement therapy. However, the limited availability of RGCs in stem cell-derived cultures makes the integration of RGCs into the host retina a complex task. Organoids offer a promising alternative to traditional branch cell-based transplantation methods.
Organoids are self-organizing three-dimensional structures that closely resemble the complexity and organization of the retina. They are generated by culturing stem cells in a controlled environment that mimics the developmental process of the retina. Recent advances in organoid protocols have made it possible to create RGCs from both human and mouse stem cells, overcoming the limitations of traditional stem cell-based transplantation methods.
Methods of Retinal Cell Replacement
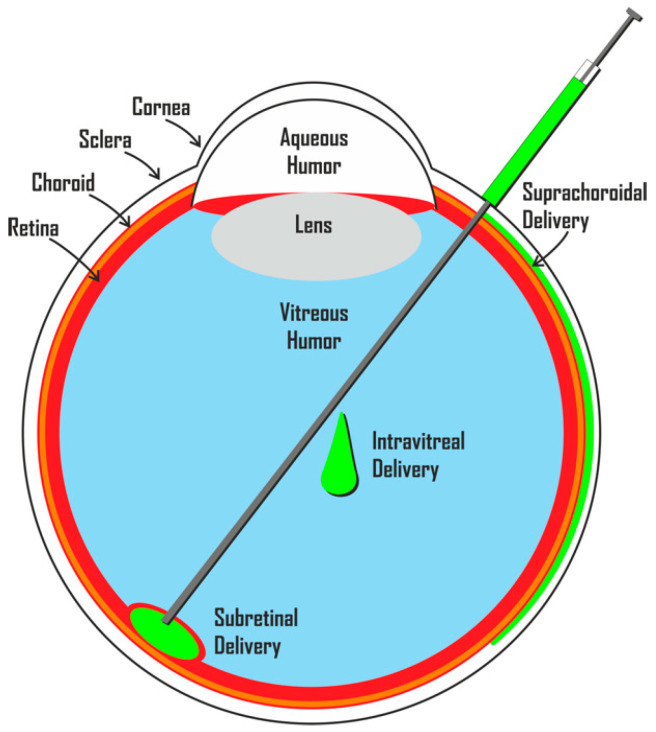
Subretinal space and vitreous cavity transplantation are two potential methods for RGC replacement. Subretinal space transplantation involves transplanting cells into the subretinal space, which is located between the retinal pigment epithelium and the photoreceptor layer. The transplanted cells can integrate into the host retina and potentially restore vision.
Vitreous cavity transplantation, on the other hand, involves transplanting cells into the vitreous cavity of the eye, which is the gelatinous substance located behind the lens. The transplanted cells can secrete neurotrophic factors that promote the survival of the remaining RGCs and stimulate the regeneration of damaged ones.
Clinical Trials Exploring Cell Therapies
Numerous studies are currently investigating the possibility of using RGC replacement therapy as a treatment for glaucoma. One such study was conducted on rats with glaucoma, and it showed significant improvement in visual function and RGC survival when RGC-like cells were transplanted into them. These RGC-like cells were differentiated from human-induced pluripotent stem cells, which can develop into any cell in the human body.
The study demonstrated that human-induced pluripotent stem cells could be a potential source for RGC transplantation in glaucoma patients. However, further research is necessary to determine the safety and effectiveness of this therapy before it can be implemented in clinical practice.
The Challenges of Making Retinal Ganglion Cells a Treatment
Still, despite the promise of studies, there are some challenges. A review done by a team in Spain assessed these challenges and more. One of the significant challenges is understanding the origin of RGCs and how to replicate their natural development in laboratory settings.
Another area for improvement is the difficulty in scaling up the production of RGCs and ensuring they can be produced in large quantities. Additionally, once produced, there are challenges in integrating and ensuring the survival of transplanted RGCs in the host tissue.
Regrowing RGC axons is also a significant challenge, as it requires identifying the factors that promote axon growth and determining how to apply them effectively. Finally, achieving functional RGC replacement is a significant hurdle, as it involves creating RGCs that can function as well as, or better than, the RGCs they are replacing.
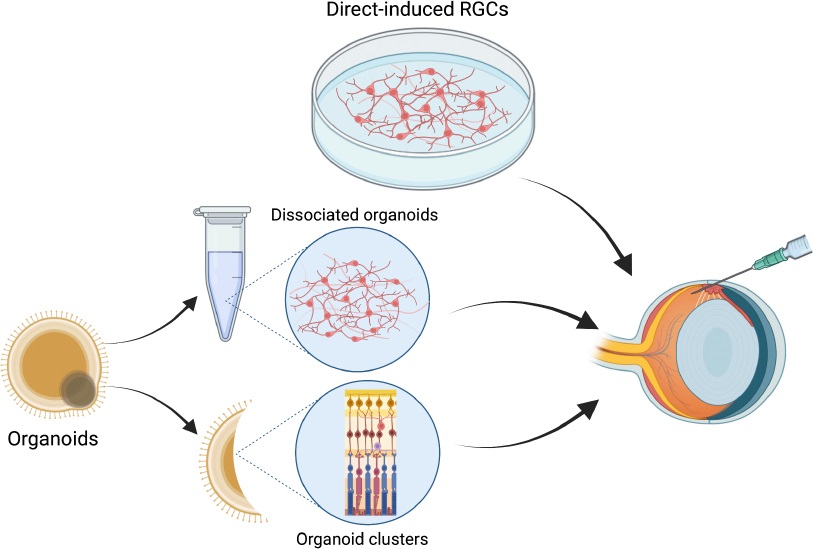
However, recent advances in stem cell technologies and organoid protocols discussed before offer new solutions to these limitations. For example, organoid protocols enable the generation of RGCs that more closely resemble those found in the human retina, thus improving the accuracy and efficacy of transplantation. Additionally, stem cell technologies have been used to create RGCs capable of integrating into the host tissue and regenerating axons.
RGC replacement therapy shows potential as a new therapeutic approach for treating glaucoma. Although there are challenges to overcome regarding scaling up RGC production and achieving reliable and functional integration, recent advancements in stem cell technologies and organoid protocols offer promising solutions. Clinical trials investigating the potential of RGC replacement therapy have reported positive outcomes, providing hope for restoring vision in glaucoma patients.
To learn more about the eye, read more stories at www.williamhaseltine.com

