Progress in Treating High Cholesterol: Clinical Trial Interim Results
(Posted on Wednesday, January 17, 2024)
High cholesterol can cause plaque buildup in the arteries. Over time, this can lead to heart attack. One clinical trial aims to lower LDL cholesterol for a lifetime with a single infusion of gene-editing RNA.
GETTY
A new, CRISPR-powered gene therapy seeks to rewrite the rules of cholesterol care. The treatment promises to obliterate ‘bad’ cholesterol with a single infusion, marking a potential paradigm shift in cardiovascular care.
A Gene for Bad Cholesterol
The balance between “good” cholesterol and “bad” cholesterol—High-Density Lipoprotein (HDL) and Low-Density Lipoprotein (LDL) cholesterol—can be influenced by various factors, including healthy eating and regular physical activity. However, for the 1 in 250 people who inherit a gene called Heterozygous Familial Hypercholesterolemia (HeFH) from their parents, diet and exercise alone cannot bring their heightened LDL cholesterol down to healthy levels. People born with this condition require daily pills called statins or periodic injections to lower their cholesterol—a major burden for both the patient and the healthcare system. Without frequent treatment, LDL cholesterol can accumulate in the arteries, significantly increasing the risk of early heart disease or heart attacks. Conversely, prompt and continuous treatment can reduce the risk of heart disease by as much as 80%.
Using CRISPR to Change Genes In-Vivo
What if this treatment regime could be simplified? What if sky-high bad cholesterol could be eliminated in a single stroke? This is the vision held by VERVE Therapeutics as their Phase I clinical trial reports interim results. The aim is to permanently lower genetically high levels of low-density lipoprotein cholesterol or “bad cholesterol” with a single infusion of a CRISPR-based gene-editing therapy. The plan relies on two scientific advances, lipid nanoparticle technology and CRISPR-based gene editing, to turn off a cholesterol-raising gene in the liver. Fascinatingly, the genetic changes don’t happen in the lab—they occur within the body. This is a stark departure from other gene therapies which require cell extraction.
The lipid nanoparticles act as a vehicle. They are designed to carry gene-editing information to specific cells. This vehicle is used to deliver the mRNA in COVID-19 vaccines and has also been adopted in experiments to treat cardiac fibrosis and sickle cell disease. Here, the nanoparticle delivers two gene-editing components to the liver, the organ responsible for managing cholesterol.
Sugar molecules called GalNAc, or N-acetylgalactosamine, decorate the nanoparticle surface and interact with LDL receptors on the liver cell. The nanoparticle enters the cell, begins to degrade, and releases the genetic material into the cytoplasm. The nanoparticle cargo ultimately targets a gene in the liver cells known as proprotein convertase subtilisin/kexin type 9 or PCSK9. Since this gene produces proteins that destroy bad cholesterol receptors in the liver, neutralizing this gene should allow the liver to process LDL cholesterol properly.
The gene-editing components originate from CRISPR-Cas9, a revolutionary system that uses an enzyme called Cas9 and a guide RNA to cut and alter DNA at a precise location. Unlike traditional CRISPR-Cas9 gene editing, This product uses an mRNA and a guide RNA that changes a single base of DNA without cutting the DNA. As the DNA does not break with this method, it is less likely to cause off-target mutations. Switching a specific adenine into guanine (“A” to “G”) should inactivate the PCSK9 gene and lower LDL cholesterol in the body.
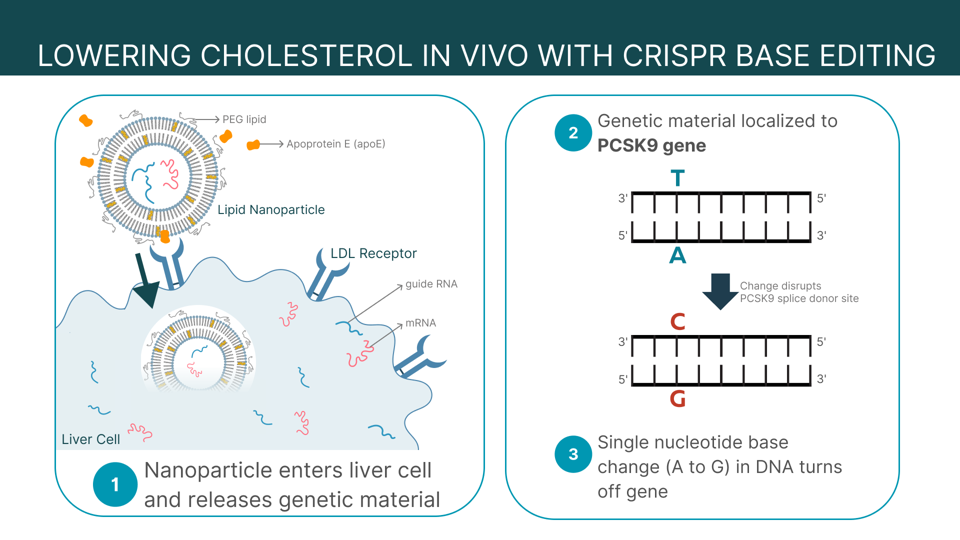
FIGURE 1: Summary of VERVE-101 lipid nanoparticle mechanism. Hepatocytes, or liver cells, uptake the lipid nanoparticles via low-density lipid receptors (LDLR). The desired mRNA and guide RNA (gRNA) inside the nanoparticle enter the cell, where they inactivate cholesterol-raising gene PCSK9 via a single-base edit from adenine to guanine (A to G). Abbreviations: apoE, apoprotein E (a protein that can package lipids and move it throughout the bloodstream); PEG lipid, poly(ethylene glycol) phospholipid.
ACCESS HEALTH INTERNATIONAL
Early Trial Results
A preclinical study of this gene therapy demonstrated promising results. The product successfully lowered up to 69% of LDL cholesterol in non-human primates for up to 476 days. Additionally, the infusion reduced up to 83% of PCSK9 protein found in the blood of non-human primates. The question remained: how would the infusion perform in people?
A clinical trial began in May 2022 to determine if the product is safe for humans to use and the highest tolerable dose. The trial recruited ten participants, two women and eight men, who had the cholesterol gene and a high risk for cardiovascular events. Notably, everyone displayed high bad cholesterol levels despite taking the maximum-tolerated medicines for lowering LDL cholesterol. The majority of participants either experienced a heart attack prior, had pre-existing severe coronary artery disease, or received a major procedure to improve the heart’s blood flow.
For the procedure, the patients were prepped with dexamethasone (a steroid used to reduce inflammation) and antihistamines. The group then split into four cohorts marked by differing doses. Each received a single intravenous CRISPR-edited infusion, with the lowest dose of 0.1mg/kg and the highest at 0.6mg/kg.
Two participants received the second-highest dose at 0.45mg/kg. The infusion reduced their bad cholesterol by 39% and 48%; the PCSK9 protein levels in the blood reduced to 49% and 59%, respectively.
Only one person received the highest dose. Their blood PCSK9 protein levels decreased by an astonishing 84%, while their bad cholesterol levels reduced by 55% and remained decreased even six months later. The study will continue to follow up with the participants. Furthermore, as required by the FDA, participant follow-ups will continue for the next 14 years to monitor the effects of the genetic change.
As for safety, the majority of observed adverse effects were mild. However, two participants experienced serious effects: a cardiac arrest, a heart attack and an irregular heartbeat. An independent data and safety monitoring board agreed that the events could be attributed to the patients’ underlying advanced coronary artery disease, and recommended the trial continue with the same dosing.
Next On the Horizon
The landscape of gene therapy is rapidly advancing. As demonstrated by this early clinical trial, the dream of changing genes within the body instead of outside it is slowly becoming a reality. Soon it may be possible to simply treat diseases that normally require decades of medication and monitoring to control. Yet, while tantalizingly close, this small trial will need to be verified with larger trials before this product hits the shelves.
Future plans for the study entail recruiting more participants in the two highest dose cohorts to complete the dose escalation phase, and initiating a randomized and placebo-controlled phase 2 trial in 2025 for their PCSK9 program. It will be exciting to watch this research unfold as more data is collected on its safety, ideal dosing and long-term efficacy.
This article was originally published on Forbes and can be read online here.

