Why Do We Age? DNA Damage A Likely Cause
(Posted on Sunday, February 18, 2024)
This story is one of many exploring recent advances in the science of longevity and aging. The following few articles in the series, including this one, will focus on the relationship between genetics and longevity; Which genes are involved in aging and longevity? How are they involved? What are the therapeutic implications?
Think of aging as a series of misfires; processes and pathways that ran smoothly while we were young slowly begin to break down. Over time, these interruptions build up, leading to the signs we’ve all come to associate with later life: loss of muscle mass, a weakening of the immune system, memory troubles, and so on. What we see “on the skin” is mirrored at the genetic level, with clear differences between younger and older adults. But until now, the exact cause of these age-related genetic changes remained poorly understood. A recent study, published in Nature Genetics, suggests DNA damage may be to blame.
The “Genetic Fingerprint” of Old Age
Genes make the world go round. That sounds like an exaggeration, but it’s not. Every single one of the processes we depend on for life is, in one way or another, shaped by genes. Remember, genes act as a blueprint for the production of proteins. And without proteins, everything grinds to a stop — they are, after all, the molecules that perform functions.
Whether a protein is produced or not, and when and where and how much of it, is carefully regulated by a process called gene expression. Essentially, gene expression is a kind of genetic “on/off” switch. When someone gets sick with a viral infection, for example, their body will start to “switch on” genes related to the immune response, thereby mobilizing the relevant immune cells to help defend against the threat. When gene expression is properly regulated, cellular functions run smoothly. But if the balance is disturbed, genes that should be “off” may remain “on”, and vice versa. Or too much or too little of a protein may be produced.
Aging is characterized by a certain pattern of gene expression. Things misfire, but they misfire in a predictable way. In a sense, old age could be said to have a specific “genetic fingerprint”. Just like a thief that leaves behind an oily fingerprint at a crime scene, age leaves its own telltale mark. And this is true across animal species, from nematodes all the way to humans.
The changes in gene expression associated with aging have been known for some time, but what prompts these changes in the first place has been surprisingly difficult to answer; we know what aging looks like at the genetic level, but we don’t know why it happens.
Genetic Alchemy: From DNA to RNA
Protein production is a complex, multi-step process. And as with any complex process involving multiple moving parts, there is lots of room for things to go wrong. Indeed, the findings of this new study suggest that changes in gene expression with age may be related to malfunctions in protein synthesis: it seems like cumulative damage to DNA (deoxyribonucleic acid) throws a monkey wrench into a crucial step called “transcription”.
Proteins are produced from RNA (ribonucleic acid). But our genes are stored in the form of DNA. So, this DNA first needs to be converted into RNA. In technical lingo, this is called transcription. Why the need for this act of genetic alchemy? Well, our DNA is stored in the nuclei of cells for safekeeping. To minimize damage, it does not leave this area of the cell. But the production of proteins happens elsewhere, in the cytoplasm. So, the genetic information needs to get from the nucleus to the cytoplasm. This is where RNA —messenger RNA (mRNA), to be exact— comes into play. Whereas DNA is used for long-term storage, mRNA acts as a shuttle of single-use genetic instructions. It encodes a copy of a particular gene and ferries it out of the nucleus into the cytoplasm, where the gene can be made into a protein. The process is similar to photocopying a section of a rare book that you need but that you are not allowed to check out of the library.
The body even has its very own genetic photocopier, called RNA polymerase II (RNAPII). This is a multiprotein complex that attaches to a specific section of DNA, depending on which gene it needs to transcribe, and then runs along the target gene and spits out a complementary RNA copy: DNA in, RNA out. The resulting RNA strand, called a transcript, is a precursor to messenger RNA.
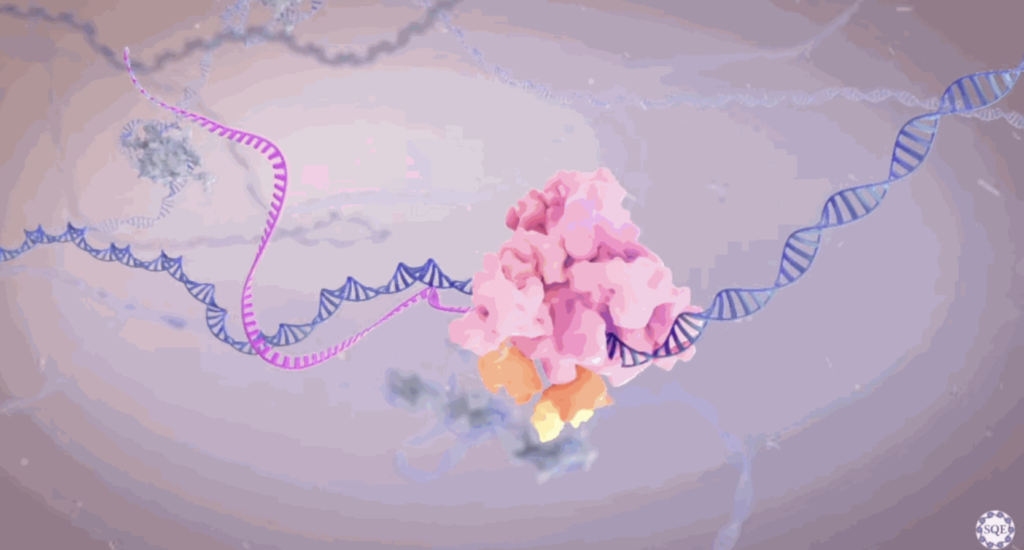
RNA Polymerase II (shown in pink and orange) moves across a strand of DNA (shown in blue) and produces a complementary RNA strand (shown in magenta). ‘LICENSING PRODUCTIVE TRANSCRIPTION THROUGH RNA POLYMERASE II STABILITY”, SHILATIFARD LABORATORY AT NU
DNA Damage Interrupts Transcription, Leads to Aging?
In this study, Akos Gyenis, Jiang Chang, and their colleagues at Erasmus University Medical Center, The Netherlands, discovered that, in older mice, RNA polymerase II often begins to stall while transcribing DNA into RNA. By analyzing the liver of two-year-old mice —ancient, by mice standards— they noticed that up to 40% of all RNA polymerase II complexes had stalled. To add to this, each stalled complex is likely to block the next three complexes behind it, quickly leading to queuing and gunking up the DNA strand until the obstruction can be cleared. The researchers found that larger genes are especially prone to these issues, leading to a bias towards expression of small genes.
With transcription interrupted, gene expression is also interrupted. As a result, many cellular pathways begin to go haywire; they are deprived of the proteins they need for problem-free functioning. These include all of the same pathways that begin to malfunction as we age. In other words, the genetic fingerprint produced by interrupted transcription is the same as that produced by aging, suggesting that the two are intimately connected. Affected pathways include those involved in nutrient sensing, clearing of cellular debris, energy metabolism, immune function, and the ability of cells to handle damage. All of these play vital roles in shaping life span.
The researchers next set out to understand what caused the RNA polymerase II to stall in older mice. Their suspicions fell on spontaneous, internal DNA damage. Gene expression patterns in cells that have been exposed to DNA-damaging agents are very similar to those seen during normal aging. Premature aging disorders, such as Cockayne syndrome, are also characterized by DNA damage; the usual DNA repair mechanisms malfunction, leading to stalled RNA polymerases at sites of damage, known as lesions. Given these similarities, the scientists speculated that DNA damage could also be involved in normal aging.
To test their hunch, the researchers monitored genetically altered mice that lacked the usual DNA repair machinery, leaving them prone to accumulated DNA damage. These mice exhibit many features of premature aging, including a significantly shortened lifespan. As expected, the rate of transcription was noticeably lower in these mice compared to healthy controls.
Implications and Takeaways
Although we have a good grasp of the way gene expression changes with age, we do not fully understand what causes these genetic changes. In a sense, it is like we have been looking at the symptoms of a disease without knowing the root cause. This new study shines a light on one of the possible mechanisms: accumulated DNA damage snags at RNA polymerase II as it tries to transcribe the template strand into RNA. When RNA polymerase II hits a spot of damage, it stalls and becomes stuck, interfering with transcription and causing a number of important cellular pathways to malfunction.
What does this all mean for you? Although there are no immediate therapeutic implications, work of this kind helps us better understand the inner workings of the aging process. The deeper our understanding, the more likely we are to develop effective pharmacological interventions. Until then, avoid behaviors that risk contributing to DNA damage, such as smoking tobacco and exposure to ultraviolet (UV) light. Planned, temporary caloric restriction may also help alleviate transcriptional stress.

