Checkpoint Inhibitors: A New Hope for Cancer Treatment
(Posted on Monday, April 22, 2024)
This article is part of an ongoing series on checkpoint inhibitors, a novel cancer immunotherapy. Here, we explore the history behind this revolutionary discovery. Future installments will delve deeper into the treatment’s mechanism and the latest research expanding the field.
Twenty years ago, people battling aggressive cancers faced limited treatment options. Then, a beacon of hope emerged with the advent of novel immunotherapies—among them a class of anti-cancer drugs called checkpoint inhibitors, which the FDA approved for the first time in 2011 for melanoma treatment.
This advance takes advantage of how our bodies recognize danger and fight cancer. The immune system has naturally integrated off switches called immune checkpoints to disrupt overactive white blood cells. The checkpoints prevent toxic shock and a wide range of autoimmune diseases caused by overzealous immune activity. Unfortunately, cancer cells can co-op this feature for their own use by turning off immune cells that could attack them.
Checkpoint inhibitors flip this switch; they block checkpoint proteins, allowing the white blood cells to recognize and effectively eliminate cancer cells. Put another way, if cancer cells press the immune system’s brakes, checkpoint inhibitors release them.
It took many years to reach this stage, to understand how medicines could boost the immune system’s inherent power to counter cancer. This article explores the backdrop behind this discovery—the landscape from which this treatment bloomed and the many milestones that brought us here today.
Laying the Foundation
Scientific interest in the immune system predates the 1900s. However, as the world entered a new century, the field of immunology experienced unprecedented growth. Scientists began to recognize and understand new intricacies of the immune system, such as the existence and role of T cells in 1967, dendritic cells in 1973 and natural killer cells in 1975. These discoveries laid the foundation for checkpoint inhibitor research in the late 1980s and beyond.
Present-day checkpoint inhibitors act on one of two immune checkpoints: cytotoxic T-lymphocyte antigen 4 (CTLA-4) or programmed cell death protein 1 (PD-1). These checkpoints share a similar timeline, with the CTLA-4 molecule identified first. Researchers James Allison and Tasuku Honjo received the 2018 Nobel Prize in Physiology and Medicine for their research on CTLA-4 and PD-1, respectively, but it is important to note that several independent laboratories contributed to the wealth of knowledge we now possess on immune checkpoints.
First Target: CTLA-4
In 1987, Pierre Goldstein and fellow researchers at the Centre d’lmmunologie en Marseille-Luminy, France, made the first breakthrough for checkpoint inhibitors. They identified cytotoxic T-lymphocyte antigen 4 (CTLA-4), an immune checkpoint protein found on mouse T cells. Although the authors noted its potential role in immune regulation, years would pass before people realized how this molecule could contribute to anti-cancer therapy.
The early 1990s marked an age of realizations. The Bristol-Myers Squibb Pharmaceutical Research Institute revealed in 1992 that CTLA-4 is expressed widely by killer T cells and activated T cells, similar to another receptor on T cells known as costimulatory receptor CD28. New evidence soon suggested that CTLA-4 negatively regulates T cell activity—a contrast from CD28, its sister receptor. Subsequent papers confirmed that CTLA-4 inhibits T cell activation, including research from the University of Toronto, the Brigham and Women’s Hospital and Harvard Medical School.
Jim Allison and Berkeley colleagues also recognized this molecule’s inhibitory nature. Their 1995 study observed that CTLA-4 and CD28 bind to the same B7 ligand on antigen-presenting cells but trigger opposite responses; the former reduces T cell function, while the latter activates T cell function. The following year, Dr. Allison and others delivered antibodies to block the molecule in animal models of cancer. Blocking CTLA-4 allowed for effective immune responses against tumor cells, providing the first proof of CTLA-4 as an anti-cancer target in living creatures.
CTLA-4 checkpoint inhibitors entered clinical testing in the year 2000. James Allison partnered with Bristol-Meyer Squibb to create ipilimumab, a fully humanized antibody treatment that blocks CTLA-4. The treatment was given to patients with metastatic melanoma, a type of skin cancer that has spread from the original tumor site. Although many patients responded to the therapy, it also elicited immune-related adverse effects for advanced melanoma patients in these early trials. Clinical testing continued in 2010 and in 2011 to confirm the treatment’s safety and efficacy. The FDA approved ipilimumab as the first checkpoint inhibitor in 2011—a remarkable breakthrough for cancer immunotherapies.
Second Target: PD-1
Tasuku Honjo and colleagues at Kyoto University chanced upon a different checkpoint protein in the 1990s. The Honjo group noticed the protein in 1992 while screening mouse T cell genes involved in apoptosis; the protein earned the name PD-1, or programmed cell death protein 1. The next year, they observed that aged mice without the PD-1 gene developed autoimmune-like symptoms, suggesting that PD-1 is involved in T cell self-tolerance. Additionally, T cells removed from knockout mice appeared more activated than those from normal mice. These discoveries hinted at PD-1’s role as a co-inhibitory molecule.
A 2001 paper noted that mice without PD-1 develop autoimmunity; clearly, this molecule negatively affected T cell activation, but was it through the same mechanism as CTLA-4, and what did this receptor bind to?
Answers to these questions surfaced in the 2000s. Those early years confirmed the existence of PD-1’s ligand: B7-H1, later relabelled as programmed death-ligand 1 (PD-L1). Studies illustrated that PD-L1 is expressed by antigen-presenting cells in tissues such as the heart and lungs, and that it can negatively regulate T cell activity; this differed from CTLA-4, which was expressed in lymphoma nodes, the bone marrow, and other tissues from the lymphatic system.
In 2004 and 2006, PD-L1 gene knockout mice provided evidence that PD-L1 binds to PD-1. During the 2010s, anti-PD-1 checkpoint inhibitors demonstrated promising results in clinical trials for patients with melanoma and other cancers. The goal of these inhibitors was to prevent PD-1 proteins on T cells from binding to their ligand, PD-L1, expressed on cancer cells. The FDA approved the first PD-1-targeting monoclonal antibodies in 2014: pembrolizumab and nivolumab.
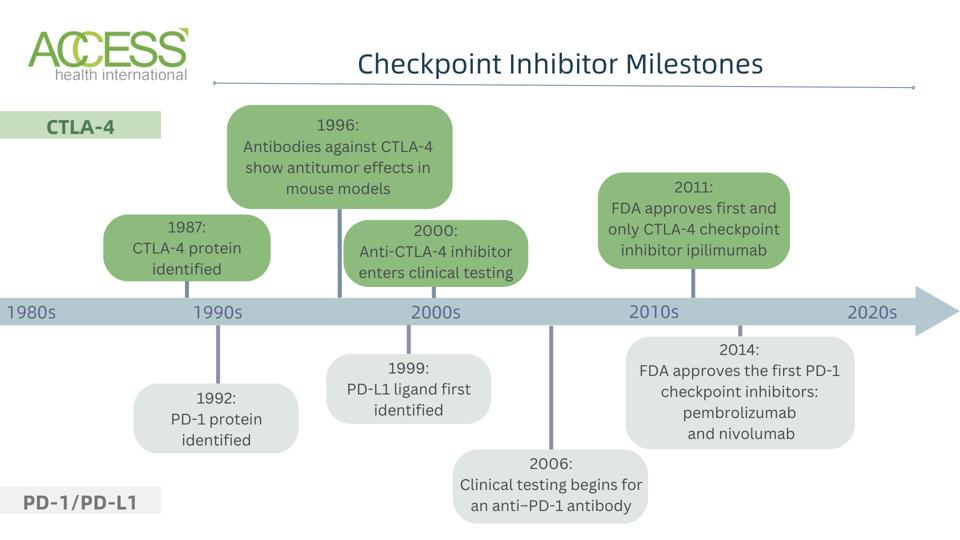
FIGURE 1: A broad timeline of milestones for CTLA-4 and PD-1/PD-L1 checkpoint inhibitors. Abbreviations: CTLA-4, cytotoxic T lymphocyte antigen 4 PD-1, programmed cell death protein 1
ACCESS HEALTH INTERNATIONAL
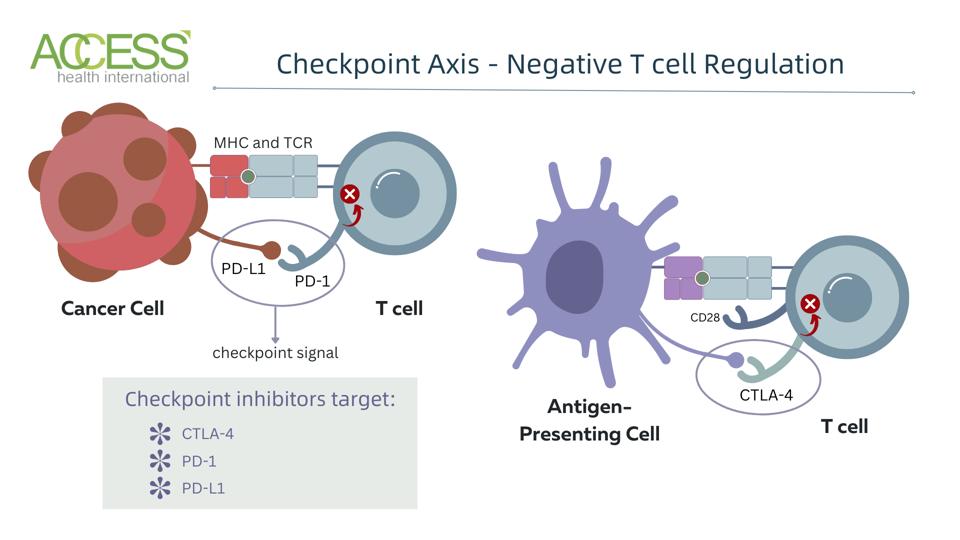
FIGURE 2: Checkpoint mechanism in T cells. Abbreviations: CTLA-4, cytotoxic T lymphocyte antigen 4 PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; MHC, major histocompatibility complex; TCR, T cell receptor
ACCESS HEALTH INTERNATIONAL
Present-Day Checkpoint Inhibitors
Decades of dedicated research have led to eight federally approved checkpoint inhibitors for 18 different types of cancer. What was once a distant dream in the early 1900s has become a testament to the profound potential of immunotherapies in the battle against disease.
Yet, efforts only continue to grow. Attention has shifted to improving checkpoint inhibitor safety, efficacy and longevity, especially through synergistic combinations with traditional cancer treatments (ex: chemotherapy). The horizon is also ever-expanding to apply checkpoint inhibitor therapy to other illnesses, such as autoimmune diseases, chronic viral infections, and neurodegenerative disorders.
This intricate dance between therapy and immune cells has more to offer. In an upcoming article, I will delve deeper into how this therapy works, shedding light on its biochemical mechanism and future possibilities.
The article was originally published on Forbes, and can be read online here.

