A New Weapon Against Cancer: Checkpoint Inhibitors, the Double-Edged Sword
(Posted on Monday, April 29, 2024)
This article joins a growing series on mono cancer treatments, including novel immunotherapies such as CAR T therapy and checkpoint inhibitors.
Cutting-edge technologies have transformed the way we treat cancers. While chemotherapy and other traditional treatments directly interfere with cancer cells, immunotherapies born in recent decades empower the body’s inherent ability to counter tumors. Checkpoint inhibitors join this refreshing charge. This novel anticancer medicine can renew the immune system’s ability to detect and attack cancer cells. By the same token, this mechanism can spark unwanted effects. To understand how this therapy works, let’s explore the immune system’s natural functions.
The immune system protects and regulates the body. This intricate network of cells, tissues and organs defends the body not only from foreign pathogens but also from itself. When a stimulus occurs, the immune system critically activates and launches an attack. Likewise, it can also downregulate the host to avoid causing severe damage.
For example, T cells are a broad group of white blood cells that perform several essential functions, including directly killing threats and recruiting other immune cells to action. However, if T cell responses become too powerful, they can have detrimental effects.
These cells can release an overwhelming bevy of immune chemicals, manifesting symptoms that range from fever to seizures, organ failure and even death. The cells may also spark autoimmune reactions by mistakenly attacking healthy tissues and cells. Alternatively, overwork could weaken the immune system; the T cells may become exhausted and lose their ability to fight threats effectively.
Luckily, the immune system possesses an embedded T cell braking system—a series of proteins called immune checkpoints.
Immune checkpoints are receptors found on the surface of T cells. Of the several checkpoints, the two most-known proteins are cytotoxic T-lymphocyte antigen 4 (CTLA-4) or programmed cell death protein 1 (PD-1). Checkpoint proteins can quiet T cells through several potential methods.
For example, CTLA-4 competes with another receptor found on T cells. These two receptors bind to the same targets on antigen-presenting cells but spark different reactions. By out-competing the other receptor, CTLA-4 prevents the T cell from fulling activating. The resulting T cell cannot execute its functions or multiply properly.
CTLA-4 and other checkpoint proteins can also bind to ligands and inhibit T cell receptor functions. T cell receptors interact with molecules—specifically major histocompatibility complexes—on antigen-presenting cells to activate T cells. When checkpoint inhibitors bind to their ligands, they can trigger a signaling cascade that downregulates key components of the T cell signaling pathway. A resting T cell cannot target enemies, produce immune chemicals or proliferate.
Exhaustion is another way to impair T cell function. If checkpoint proteins such as PD-1 are expressed for an extended period of time, the T cells can become dysfunctional and potentially undergo cell death.
Finally, researchers believe that some checkpoints can enhance the suppressive activity of regulatory T cells, a subset of immune cells that notoriously dampen T cell responses.
Immune checkpoints are designed to protect, but they can also be wielded for harm. Cancer cells manipulate immune checkpoints for their own gain. They upregulate checkpoint ligands to prevent T cells from launching a counterstrike. Just as a thief sneaks past security systems, this clever exploit allows cancer cells to evade immune detection and grow unchecked.
Checkpoint inhibitors are a class of drugs designed to counter this mechanism. When cancer tries to suppress the immune system, checkpoint inhibitors flip the switch. These inhibitors introduce antibodies into the bloodstream that interfere with immune checkpoint interactions.
As the name suggests, anti-CTLA-4 inhibitors block the CTLA-4 receptor on T cells, leaving the other receptor to bind freely. This action allows for enhanced T cell activation and proliferation. This same mechanism can sometimes deplete CTLA-4-expressing regulatory T cells in the tumor environment, knocking down another immune-suppressing agent.
Checkpoint inhibitors can also reinvigorate exhausted effector T cells. The antibody can prevent checkpoint PD-1 from interacting with its ligand, Programmed Cell Death Ligand 1 (PD-L1).
Flipping the switch can produce remarkable results. Checkpoint inhibitors successfully treat various types of cancer, including melanoma, lung cancer, and bladder cancer. Patients can experience prolonged survival and improved quality of life.
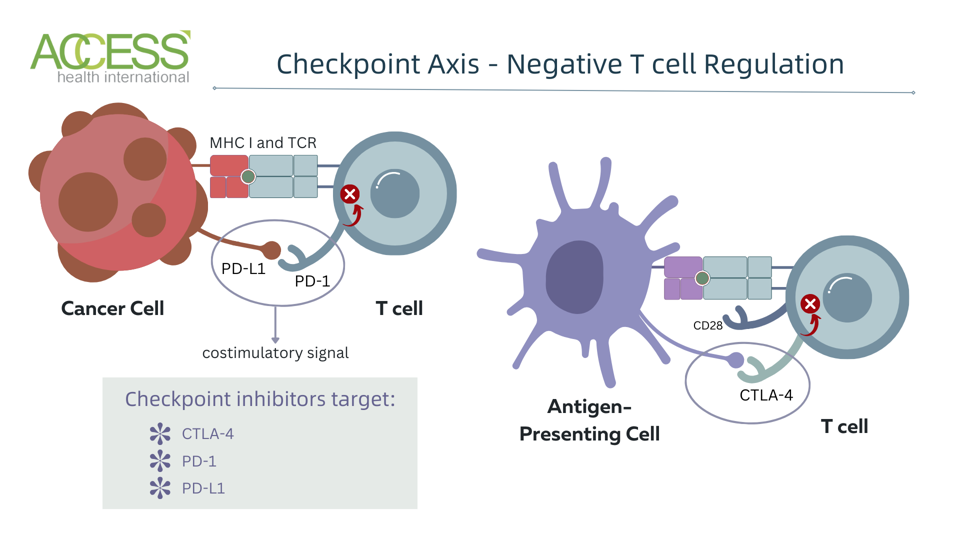
Checkpoint inhibitors release the breaks placed on white blood T cells. This immunotherapy blocks checkpoint proteins, such as CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), PD-1 (programmed cell death protein 1) and PD-L1 (programmed cell death ligand 1). These proteins normally act as ‘off switches’ that inhibit T cell activation and function. Blocking these proteins frees the T cell to activate and target cancer cells more effectively.
ACCESS HEALTH INTERNATIONAL
Takeaways
Checkpoint inhibitors embrace a game-changing approach to cancer care. These drugs slash the shackles that restrain immune cells, releasing the retaliating force cancer cells attempted to avoid. However, much like a double-edged sword, while checkpoint inhibitors can be sharpened to empower the immune system, their razor-edge can carry unintended consequences. People who take these inhibitors must be monitored carefully to manage any adverse reactions.
More about this new weapon against cancer is soon to come. Future installments in the series will delve into possible complications, innovative combinations with other cancer treatments, and further insights into optimizing their effectiveness in the battle against cancer.
Read the original article on Forbes.

