Egypt’s Swift Elimination of Hepatitis C: A Model for Success Globally
(Posted on Sunday, August 13, 2023)
Hepatitis C is a serious disease that affects more than 58 million people globally.1 Although endemic in many countries, it is possible to eliminate the disease within entire populations. Unfortunately, this has only been done in a limited number of countries. Here we analyze the successes of those countries and what it takes to eliminate hepatitis C on a national scale. The first of these requirements is the scientific means and biomedical tools necessary to detect and treat both those who are actively infected and those at risk. Next is the political will, popular support, and leadership for the meticulous implementation of those tools. Third is the organization and existence of a health system capable of diagnosing, treating, and conducting follow-ups with the entirety of the population. Finally, the cost of diagnostics and drugs must be affordable for both the government and the citizens. All these components are needed for a successful program.
Hepatitis C is a blood borne viral infection that affects the liver by causing inflammation and swelling of liver tissues. Acute infections with this virus are generally asymptomatic, but when left untreated, approximately 80% of those acute infections will develop into chronic infections. Of cases that reach chronic status, up to 20% will develop cirrhosis within 10-15 years of initial infection. For cases that progress to the cirrhosis stage, 20% will develop cirrhosis-related liver failure, and an estimated 3-5% of cirrhotic liver cases will progress to hepatocellular carcinoma.2
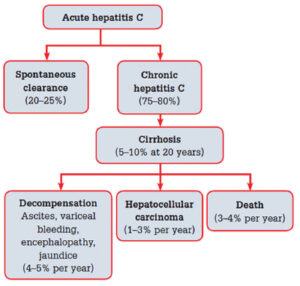
FIGURE 1: Natural history of hepatitis C virus
Source: www.racgp.org.au/afp/2013/july/hepatitis-c
The diagnostic process for hepatitis C includes two steps. The first step is to screen for exposure to the hepatitis C virus. Exposure is determined by measuring the presence of hepatitis C antibodies in the blood, which are produced whenever a person is infected. After confirmation that hepatitis C antibodies are present, an RNA test is performed to determine if there is active virus replication. This test uses a polymerase chain reaction to measure the levels of hepatitis C virus RNA in the blood. An additional PCR test may also be performed to determine the viral genotype, which plays an important role in deciding what drugs to use. There are currently six different hepatitis C genotypes present across the globe. In the United States, genotypes 1, 2, and 3 make up over 98% of infections.3
Acute HCV infection is easily detected in the body and readily triggers the body’s innate immune response. In the innate response, the immune system releases natural killer cells (NKs), proinflammatory cytokines, and the interferon-stimulated gene response (IGE), which work to interfere with HCV replication and kill off infected hepatocytes. The innate response is often not enough to curb the replication of the hepatitis C virus in liver tissue, but it allows the body time to mount an adaptive immune response. In the adaptive immune response, the body generates B-cells to induce the production of broadly neutralizing antibodies. These specialized antibodies are useful because they can recognize and block viral entry across multiple strains of hepatitis C. The immune system also releases two types of T-cells: helper CD4+ cells, which prompt B-cells to make antibodies, and cytotoxic CD8+ cells, which kill cells already infected with the hepatitis C virus.
Interestingly, up to 30% of hepatitis C infections will clear independently.4 Research about these spontaneous clearance cases reveals the importance of having robust and sustained B-cell and T-cell responses. This research is especially useful as scientists work to develop an effective vaccine.
Source: www.sciencedirect.com/science/article/abs/piiB9780128032336000175
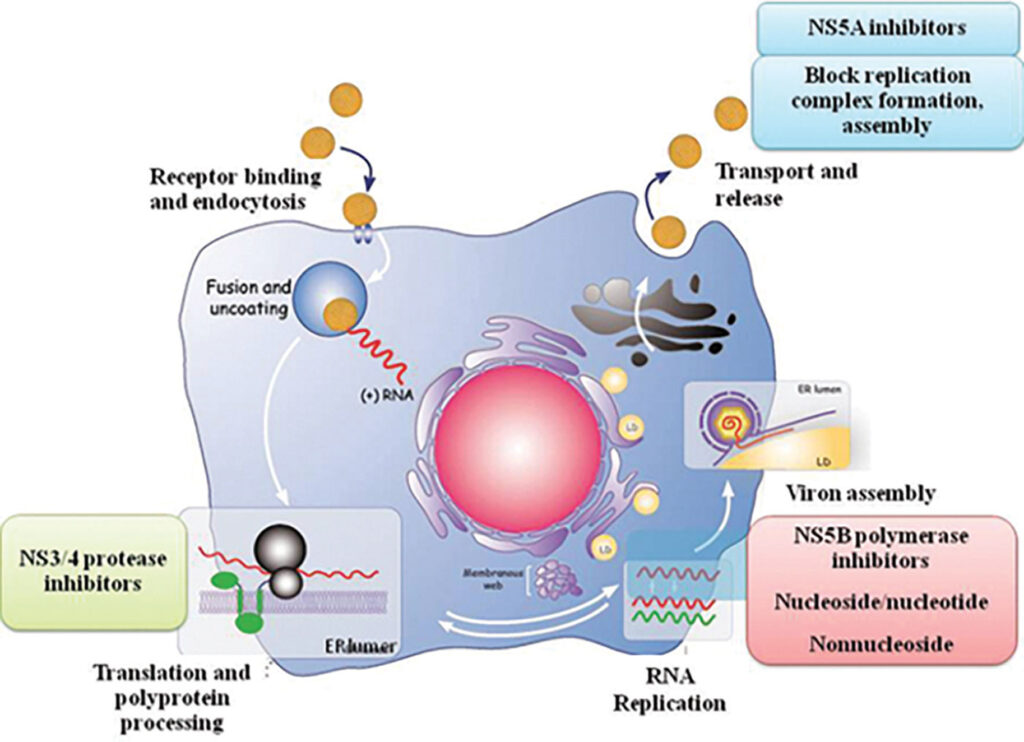
In the absence of a vaccine, hepatitis C is still highly treatable. The first generation of hepatitis C drugs focused on boosting the body’s immune system response using interferon injections. These injections, however, were susceptible to disease relapse and came with many side effects. Soon after, treatment evolved, and long-acting pegylated alpha interferons in conjunction with ribavirin became the new standard. Through pegylation, scientists increased the molecule size of the interferons to slow down the rate at which the drug was absorbed, thus increasing the amount of time the drug remained in the body. While this combination of drugs was a step up from interferon monotherapy, there were still numerous side effects, and treatment efficacy varied greatly by genotype. In the early 2010s, a new generation of drugs, called direct-acting antivirals, became available. Instead of boosting immune responses, these drugs were designed to attack specific molecular targets known to play a role in the replication of the hepatitis C virus. There are three classes of direct-acting antivirals: inhibitors of the NS3/4A protease, those that target the NS5A replicase factor, and those that target the NS5B RNA polymerase. Most direct-acting antiviral regimens consist of taking a daily pill for an average of three months, with interval RNA testing to assess how well the treatment works. A hepatitis C virus RNA level under 25 IU/mL at least three months after completing therapeutic treatment is considered a clinical cure, and at least 95% of patients who finish a course of direct-acting antiviral therapy achieve this status.5
Hepatitis C has been eliminated from some low-income countries, including Egypt and Georgia. These countries have demonstrated that it is possible to eliminate this disease in low and high-income countries, and that it can be done relatively inexpensively compared to other medical issues. If we look at the success of Egypt, we can determine that the most important part of eliminating a disease nationwide is the political will to do so. When President Abdel Fattah el-Sisi and the Egyptian government recognized the burden of hepatitis C within its borders, they committed to solving it. In September 2019, the Egyptian government implemented its 100 Million Healthy Lives program. The initiative was financed by a $250 million loan from the World Bank, supported by President el-Sisi, and managed by the Ministry of Health and Dr. Hala Zaid. The seven-month program was one of the world’s largest public health screening campaigns and allowed Egypt to efficiently screen and treat its population over the age of 12 for hepatitis C.

Source: www.hu.edu.eg/100-million-healthy-lives-campaign/heliopolis-university/
Along with political will, a successful national eradication program needs the collective support of the people, the government, and the healthcare system. Explicit government commitment can generate popular consent by generating buy-in for scientists and public health officials to create new policies and infrastructure. As we’ve seen with COVID-19, government commitment and promotion can generate public support by encouraging citizens to participate in the program. To gain popular consent for the 100 Million Healthy Lives Program, Egypt’s Ministry of Health flooded the country with advertisements for the campaign: newspapers, TV and radio stations, social media platforms, billboards, and posters featuring President el-Sisi.
Source: www.ncbi.nlm.nih.gov/pmc/articles/PMC8087425/#B35
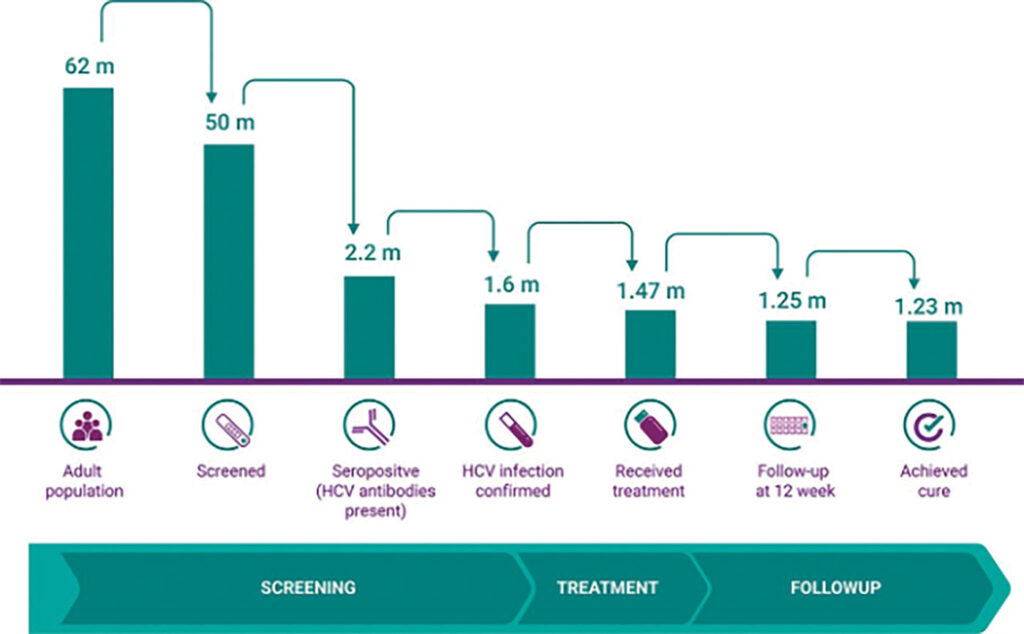
Once the political will and popular consent have been established, there must be a health system capable of reaching everybody in the country. Egypt, fortunately, has a central health system that enabled the 100 Million Lives campaign to reach its entire population. Its program followed a simple cascade of individuals from screening to post-treatment follow-up. The government started by identifying and recruiting its screening population. The first phase targeted citizens 18 years and older but later expanded to include those over the age of 12. The next step was to inform citizens about the program and encourage them to participate. In addition to a widespread promotional media campaign, citizens were encouraged to register for appointments on the centralized campaign website, www.stophcv.eg, and get tested at one of thousands of testing centers and mobile units stationed around the country. During their principal appointment, citizens were given an antibody test to identify viral exposure. Those with positive antibody results were then referred to specialized testing centers for PCR testing to confirm an active infection. Within 30 days of confirmed infection, the government was able to provide those individuals with three months of free treatment.6 Finally, citizens were retested at the end of their treatment regimens and provided a certificate of cure if found to be negative for infection. Throughout the program, 1.6 million adults tested positive for hepatitis C infections, 1.47 million accepted government-provided treatment at no cost, and an estimated 1.23 million Egyptian citizens with active hepatitis C infections were cured.7
For a large-scale screening and treatment program to work, the cost of diagnostics and drugs must be affordable to both the government and the people. Egypt was so successful in its price-lowering efforts that it was able to provide free testing and treatment for its entire population. Lowering these costs can be accomplished through negotiations or, in Egypt’s case, patent agreements. To lower the costs of its program, Egypt first declared the financial burden of distributing hepatitis C drugs as a contributor to its endemic status. This exempted the country from drug patent protection in accordance with the Trade-Related Aspects of Intellectual Property Rights agreement. Egypt was then free to purchase active pharmaceutical ingredients for seven different direct-acting antivirals from India. Egyptian pharmaceutical companies then formulated these materials for a fraction of the market price, which can be as high as $84,000 in some high-income countries.8 This reduction in costs allowed the program to provide free treatment for all its citizens at a cost of just $45 USD to the Egyptian government. These efforts also allowed those who opted for private treatment to pay just $70 USD. If Egypt can successfully lower prices, there’s no reason the United States and other high-income countries should be paying 10–100 times more for the same tests and drugs. Some countries are already designing solutions to lower costs. The United States recently announced that the National Hepatitis C Elimination Program supports the development of a single diagnostic test in addition to a pharmaceutical subscription model, which would substantially streamline and lower the total cost of testing and treatment.9
The final element of a successful eradication program is its meticulous execution. The 100 Million Healthy Lives campaign was made possible by the dedication of political and healthcare stakeholders at all levels, including Wahid Doss, MD, who oversaw the program’s implementation. Egypt’s campaign employed over 60,000 physicians, nurses, data analysts, and newly trained volunteers, many of whom had no medical background.10 The government also used a virtual private network within its national healthcare service to report all the campaign results in real time. Program officials could track how many people were screened, the age distribution, and the population percentage covered. The efficiency of the central organization system also allowed screening for other public health diseases in Egypt, including hypertension, diabetes, and obesity.
Although it is possible to design and execute a national program to eliminate hepatitis C, there are other reasons for optimism in eradicating it.
There is currently no vaccine for hepatitis C, but the ability to protect populations from getting the disease is important for curbing the incidence of new infections. Insights from spontaneous clearance cases and several other vaccines, like the pan-genotypic COVID-19 vaccine, have helped scientists develop promising methods for a hepatitis C vaccine. Research has shown that broadly neutralizing antibodies and robust and sustained T-cell responses are required to clear the disease. One promising method scientists use to induce the production of T-cells and neutralize antibodies is targeting conserved epitopes. An epitope is a specific part on the surface of a virus that T-cells can bind to and signal B-cells to produce hepatitis C antigen-specific antibodies. Conserved epitopes are T-cell targets that appear across multiple strains of hepatitis C which would allow the vaccine to work against a breadth of antigen variants.
With the incidence of hepatitis C rapidly increasing and almost half of United States citizens unaware of their infection status, the United States and other countries must act fast.11 While many countries possess biomedical science and are starting to recognize the urgency of the epidemic, it is important to note that if these programs are deficient in any one of these: the political will, popular consent, an effective healthcare system, low prices for drugs and diagnostics, or meticulous implementation, they are unlikely to succeed. The United States has unveiled a national plan to eliminate Hepatitis C within 5 years. While the U.S. has the tools to diagnose and treat its citizens, the fractured healthcare system and high drug costs make implementation and wide distribution very challenging.
While the U.S. has different mechanisms for determining drug prices than Egypt, the Inflation Reduction Act empowers the federal government to negotiate prices for certain drugs covered by the Medicare program. However, this is limited to the Medicare population and initially encompasses only ten drugs. The U.S. should explore broader and more comprehensive approaches to reduce healthcare costs for all Americans.
Even though many countries face these same barriers, it is still possible to eliminate the disease. It’s not the tools and expertise that these countries lack, it’s the political will and sustained funding. If it can be done in Egypt, it can be done in the United States, Europe, and around the world.
Read More
- www.researchgate.net/publication/280987023_T-lymphocyte_senescence_and_hepatitis_C_virus_infection
- www.researchgate.net/publication/280987023_T-lymphocyte_senescence_and_hepatitis_C_virus_infection
- www.ncbi.nlm.nih.gov/books/NBK554898/
- www.onlinelibrary.wiley.com/doi/abs/10.1111/jvh.12866
- www.cdc.gov/mmwr/volumes/71/wr/mm7132e1.htm
- www.nejm.org/doi/10.1056/NEJMsr1912628?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
- www.ncbi.nlm.nih.gov/pmc/articles/PMC8087425/#B35
- www.accessh.org/interviews/interview-with-wahid-doss-on-the-100-million-healthy-lives-project/
- www.statnews.com/2023/06/29/hepatitis-c-cure-access/
- www.nejm.org/doi/10.1056/NEJMsr1912628
- www.cdc.gov/hepatitis/statistics/surveillanceguidance/HepatitisC.htm
Read the original article on Inside Precision Medicine.

