Tackling Hard-to-Treat Tumors
(Posted on Tuesday, July 9, 2024)
Solid tumors are notoriously difficult to suppress. They survive and thrive in the body despite best efforts to control them—resisting even the latest immunotherapies such as PD-1 and PD-L1 targeting checkpoint inhibitors. One of the ways researchers are trying to address this problem is by disarming the tumor’s defenses against the immune system. This article describes one such effort: a novel antibody that could reinvigorate antitumor responses in patients with treatment-resistant tumors.
Targeting Checkpoints on Tumors
Patients with solid tumors may be offered a treatment called checkpoint inhibitors. This class of anticancer drugs uses antibodies to release the immune system’s inherent ability to fight cancer. The antibodies block inhibitory proteins called immune checkpoints found on the surface of specific immune cells and cancer cells. This interaction reinvigorates T cells, allowing them to recognize and attack cancer cells more effectively.
A significant issue with these drugs is resistance. In these cases, checkpoint inhibitors may never elicit a response in the patient. Alternatively, the treatment may prove initially beneficial but become less effective over time. This can occur due to changes in the tumor environment, an upregulation of different tumor checkpoint proteins, and other factors. Developing strategies to overcome resistance will be vital to unlocking the full potential of checkpoint inhibitors and improving outcomes for patients battling solid tumors.
How the Experimental Antibody Works
Researchers at the Vall d’Hebron Institute of Oncology in Spain have developed an antibody that may offer renewed hope for people who have undergone more than one unsuccessful cancer treatment. Early clinical results show that their FS222 antibody can disarm tumors even when other anticancer options falter.
This antibody functions distinctly from the antibodies found in checkpoint inhibitors. Inhibitors bind solely to checkpoint proteins, such as PD-1 found primarily on white T cells, or PD-L1, its binding partner on tumor cells. The interaction blocks other proteins from interacting with the inhibitory checkpoint.
In contrast, the experimental antibody is bispecific; it binds to proteins on tumor cells and on T cells. More specifically, it tightly binds to PD-L1 checkpoint proteins on one side and to a co-stimulatory receptor on T cells on the other. This antibody prevents checkpoint protein interactions and encourages the T cell to activate and retaliate against tumors through the T cell co-stimulatory receptors.
Selectively activating the immune system against tumors in this way could reap particular benefits for patients who have failed anti-PD-1 blockade therapy. The treatment could deliver a two-fold punch by simultaneously targeting a PD-1 partner molecule and riling up T cells.
The highly specific binding also helps ensure the costimulatory receptor activation occurs primarily in the tumor microenviornment where PD-L1 checkpoints are expressed. This should reduce the risk of systemic immune activation and potential unwanted effects.
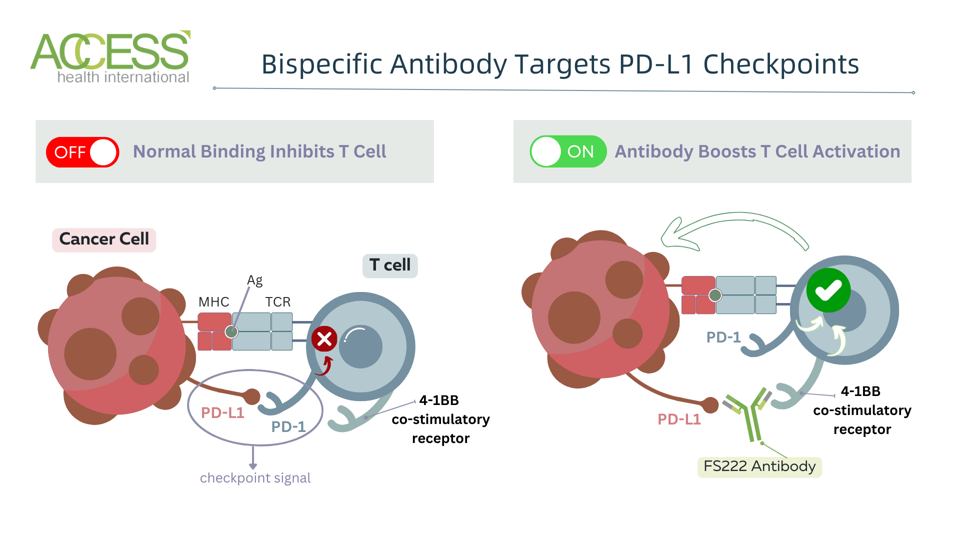
Figure 1: Experimental antibody mechanism. The antibody contains four total binding sites—two bind to PD-L1 checkpoints on tumor cells, while the other two bind to 4-1BB co-stimulatory receptors on T cells. [Abbreviations: MHC, major histocompatibility complex; Ag, antigen; TCR, T cell receptor; PD-1, programmed cell death protein; PD-L1, programmed cell death ligand 1]
ACCESS HEALTH INTERNATIONAL
Antibody Safety Is Tolerable
The primary purpose of the trial was to determine the safety and dosing of the experimental antibody. For this, over 100 patients with various advanced solid tumors were enrolled in the study, including melanoma, non-small cell lung cancer, ovarian cancer, triple-negative breast cancer and colon cancer. All participants had previously undergone at least one cancer treatment that did not succeed.
The patients were given increasing doses of the experimental antibody every three or four weeks. Treatment was discontinued if the patient’s condition worsened—for example, if the tumor grew in size or spread—or if the antibody elicited unacceptable toxicity. These early results reflect the outcomes of only 90 patients; they were exposed to the antibody for a median average of 82.5 days.
Overall, the antibody demonstrated a tolerable and manageable safety profile. Patients commonly reported fever and a general lack of energy and strength. The treatment also elevated liver enzymes and decreased levels of platelets and neutrophils, a type of white blood cell.
Five patients experienced febrile neutropenia, a severe condition marked by fever, abnormally low neutrophil count and high risk of infection. Other less common but dangerous symptoms include fever and cytokine release syndrome, an inflammatory condition often produced by some immunotherapies such as CAR T therapy.
Promising Antitumor Results for Melanoma
Beyond its safety profile, the antibody also illustrated encouraging antitumor responses. The treatment produced an overall response rate of almost 16% across all tumor types in the study, including ovarian cancer, colon cancer and breast cancer. However, the antibody elicited the most significant responses in patients with advanced melanoma.
Cutaneous melanoma is a common and aggressive skin cancer that starts in pigment-producing cells. Anti-PD-1 checkpoint inhibitors are a viable treatment option for people with advanced and spreading forms of this cancer, but the tumor can return. In the trial, the experimental antibody elicited responses in 60% of these patients who have previously tried PD-1 checkpoint inhibitors—a marked increase compared to the other tumor types. Moreover, the cancer was controlled in over 85% of this cohort. These results offer an optimistic future for patients who have experienced relapse after trying checkpoint inhibitors.
Looking Ahead
Solid tumors resist many anticancer treatments and can render them ineffective, as is the case with checkpoint inhibitors. The experimental antibody featured in this trial provides a novel solution for those whose cancer has relapsed after treatment, with particular benefit for patients with advanced melanoma. It will be exciting to watch as the study continues to evaluate the therapy’s efficacy with a larger participant population.
This article joins a growing series on mono cancer treatments, including novel immunotherapies such as CAR T therapy and checkpoint inhibitors. Find more at www.williamhaseltine.com.

