How Cancer Immunotherapy Checkpoint Inhibitors Work
(Posted on Monday, July 29, 2024)
Skin cancers, lung cancers, lymphomas—though these cancers affect different parts of the body, they can all be treated with one of the latest therapies medicine has to offer: checkpoint inhibitors. This family of anticancer drugs can treat over 25 different types of advanced tumors and can be combined with other cancer treatments such as chemotherapy and radiation.
Perhaps most fascinating is how this therapy works. With each intravenous infusion, antibodies rush into the bloodstream. They block proteins called immune checkpoints and restore the immune cell’s innate ability to kill tumors. This article outlines how both immune checkpoints and checkpoint inhibitors work. Although complicated at first glance, this process is critical to understanding how inhibitors can fight tumors.
Balance in the Immune System
There is a natural balance in the immune system. While it must be activated to retaliate against threats, it is also necessary to restrain the immune system to avoid overreaction and allow the immune system to recognize new issues as they arise. This means the immune system can send signals to rile up and slow down immune cells.
Activating T Cell Signals
The immune system engages a fleet of different immune cells to counter threats. One group known as T cells plays an important role by launching pointed attacks.
With the help of other immune cells, T cells undergo a process called T cell activation. This process relies on two cell signals—the main signal from its T cell receptor and a second crucial signal from a co-stimulating receptor—to trigger a waterfall of cell changes. Through activation, the cells learn how to recognize specific proteins found on tumors and act accordingly; for helper T cells, this means recruiting other cells to attack. Killer T cells, in contrast, can release cytotoxic chemicals to eliminate their targets. Activation also encourages the cell to multiply and survive.
Checkpoints Stop T Cell Signals
To counter overactive immune cells, the immune system relies on proteins called immune checkpoints to slow down cellular activity.
Checkpoint proteins can be found on the surface of several cells, including immune T cells. The protein interacts with a partner receptor on another cell and begins to change shape. The change sets off inhibitory chemicals in the T cells—chemicals that interfere with the cascade of signals T cells need to activate, proliferate and survive.
Not all checkpoint proteins trigger the same signal chain reaction. Let’s consider a checkpoint protein called cytotoxic T cell lymphocyte-4, or CTLA-4. This checkpoint protein binds to the costimulatory receptors that normally help activate the T cell. In fact, the checkpoint binds more tightly to the receptors than other proteins. This means the checkpoint prevents cell activation both by sending inhibitory signals and out-competing other molecules for the costimulatory receptors.
Figure 1 illustrates how this checkpoint disrupts normal T cell activation. After binding to a costimulatory receptor, the CTLA-4 checkpoint is tagged with a phosphate group. The movement of phosphate groups is important. Generally, adding a phosphate group activates proteins and turns them “on,” while removing a phosphate group turns the protein “off.”
Here, the phosphate group attracts an enzyme called SHP-2 to the checkpoint. The enzyme then interrupts signals needed for T cell activation, such as CD3, by removing phosphate groups from these proteins. As a result, the T cells become less active, produce fewer immune signals, and are less involved in immune responses and inflammation. CTLA-4 checkpoints also affect the cell cycle and reduce the activity of key transcription factors such as activator protein 1 (AP1), nuclear factor of activated T cells (NFAT) and NF-κB, which are proteins important for T cell activation and function.
Figure 1: CTLA-4 Signal Pathway. When CTLA-4 checkpoint proteins bind to CD80/86 costimulatory receptors, they release chemicals such as SHP2, SYP and PP2A. These chemicals interrupt the cascade of signals needed for a T cell to activate (Shown in red). [Abbreviations: APC, antigen-presenting cell; MHC, major histocompatibility complex; TCR, T cell receptor].
“CURRENT UNDERSTANDING OF CTLA-4: FROM MECHANISM TO AUTOIMMUNE DISEASES,” HOSSEN MM, MA Y. (2023).
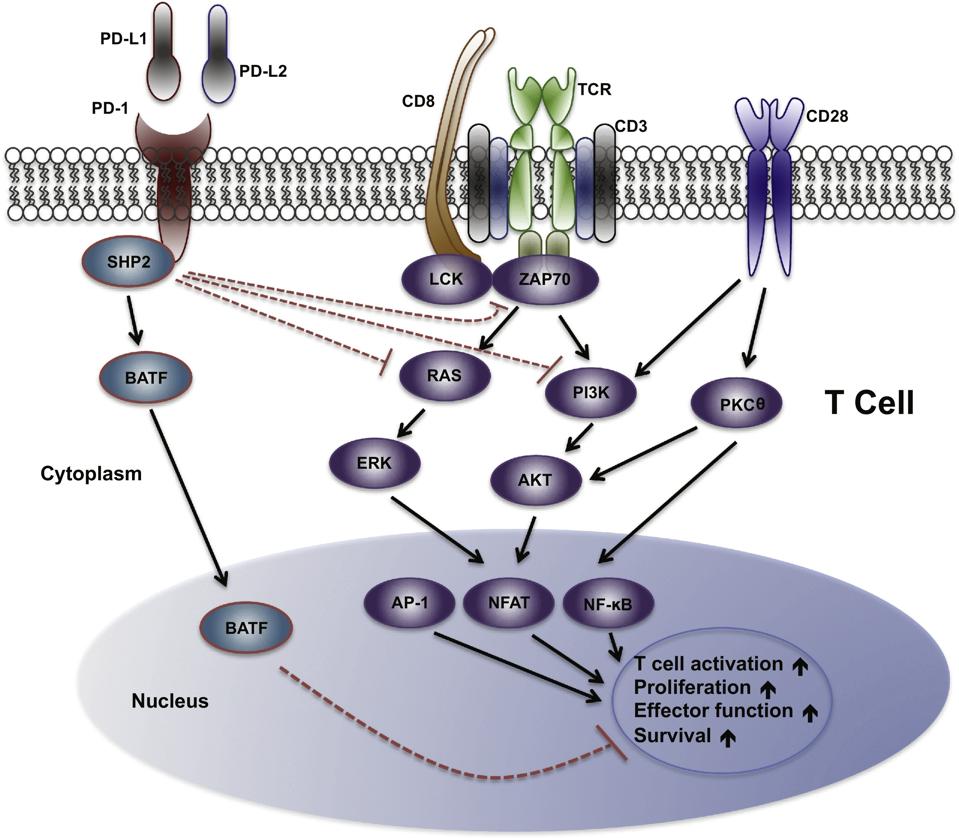
Another well-studied checkpoint pathway involves PD-1, or programmed cell death protein-1. This checkpoint can interact with two partner receptors: programmed cell death ligands 1 and 2, known as PD-L1 and PD-L2. Upon binding, the PD-1 checkpoint gains a phosphate molecule. The phosphate group then attracts enzymes such as SHP-2, which turn off certain signals within the cell. Specifically, SHP-2 reduces the activity of signals from the T cell receptor (TCR) and another molecule called CD28, both of which are important for activating T cells.
Dampening these signals decreases the activation of various pathways in the cell, including ZAP70, RAS and PI3K, as illustrated in Figure 2. This pathway affects proteins needed to regulate the expression of genes that influence T cell activation and function: NF-κB, AP-1, and NF-AT. The T cell ultimately produces fewer signaling molecules, divides less frequently and is more likely to die.
Figure 2: PD-1 Signal Pathway. PD-1 checkpoints interrupt signals a T cell needs to activate, proliferate, gain function and survive (shown with red dotted arrows).
“APPLICATION OF PD-1 BLOCKADE IN CANCER IMMUNOTHERAPY,” WU ET AL. (2019).
Cancer Cells Hijack Checkpoints
Activation helps T cells suppress tumors and other dangers. However, cancer cells are nebulous opponents. They can warp their appearance and sneak past immune cells by hijacking checkpoint proteins. By expressing checkpoint partner receptors such as CD80, PD-L1 or PD-L2 on their cell surface, cancer cells can subdue immune cells that would otherwise retaliate. In essence, what was once a safety measure for the immune system turns into a vehicle for tumor growth.
Enter Checkpoint Inhibitors
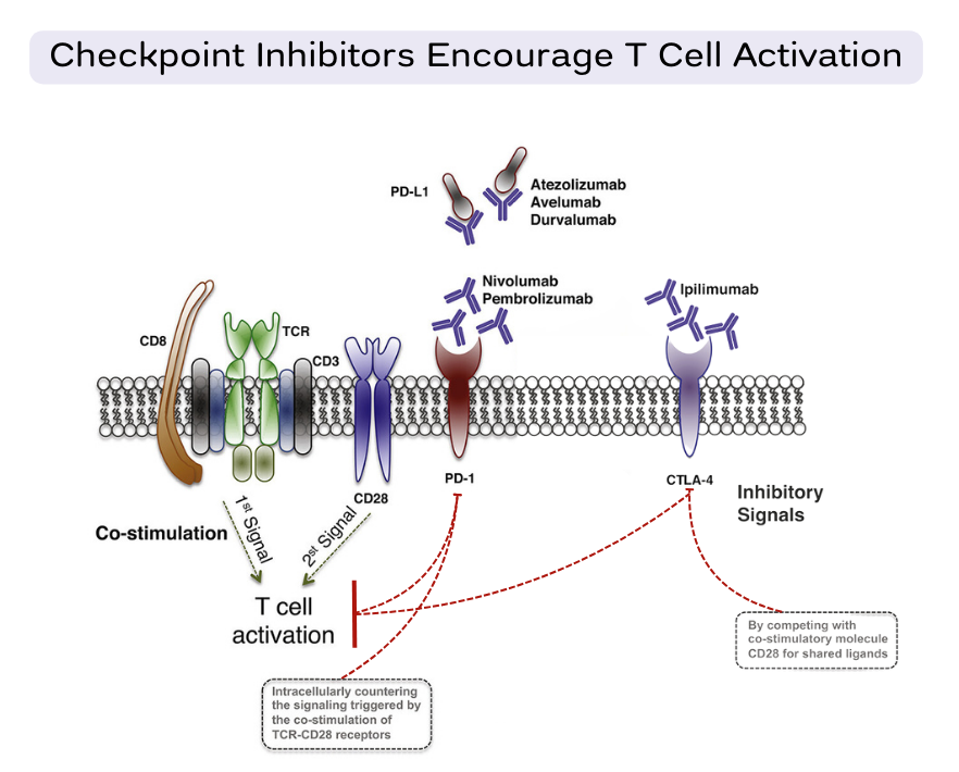
When tumor cells threaten the immune system’s balance, we can tip the scales back in our favor with checkpoint inhibitors. These immunotherapy drugs are designed to lift the restraints placed on immune cells. The drug’s antibodies bind to checkpoint proteins before they can interact with their partner receptors. With the checkpoint blocked, the activation signal cascade can flow unobstructed, allowing the T cell to freely activate and recognize tumors. Figure 3 showcases how currently approved checkpoint inhibitors block one of three immune checkpoints: CTLA-4 or partner receptors PD-1 and PD-L1.
Figure 3: Checkpoint inhibitor blockade. The antibodies used in checkpoint blockades prevent immune checkpoint proteins such as CTLA-4, PD-1 or PD-L1 from sending inhibitory T cell signals (shown in red). The T cell is then free to activate and retaliate against tumors.
ADAPTED FROM: “APPLICATION OF PD-1 BLOCKADE IN CANCER IMMUNOTHERAPY,” WU ET AL. (2019).
Takeaways
The body can orchestrate cell activity to achieve immune balance. However, cancer cells can skew this delicate equilibrium using the immune system’s own mechanisms. In these cases, we can use checkpoint inhibitors to help restore the immune system’s functions and effectively counter tumors that use immune checkpoints to grow undetected. As research continues to uncover the intricacies of checkpoint signaling, new therapies may emerge that can further enhance this immune response against even the most resilient cancers.
Read the original article on Forbes.