We’re Making Exciting Progress In Developing Covid-19 Drugs
(Posted on Tuesday, June 30, 2020)
BRAZIL – 2020/06/24: Pharmaceutical pills displayed on a table with a COVID-19 inscription on the background. (Photo Illustration by Rafael Henrique/SOPA Images/LightRocket via Getty Images)
SOPA IMAGES/LIGHTROCKET VIA GETTY IMAGES
Drugs specifically designed to prevent and treat Covid-19 are urgently needed. The good news is they are on their way.
The winning strategy for several other viral diseases proved to be drugs that inhibit virus replication in patients. Drugs that treat HIV/AIDS, hepatitis C, and herpes simplex type 1 and 2 are well-known examples. These drugs work by blocking the action of proteins the virus needs to reproduce itself.
The most common targets are the enzymes a virus needs to copy their genome, the polymerases, and those they need to cut larger proteins into smaller functional fragments, the proteases. The discovery of anti-HIV proteases, combined with an anti-polymerase, paved the way for long term survival in people infected by HIV-1. Similarly, it was the discovery of anti-protease drugs that enabled the curative treatment of hepatitis C infections.
The virus that causes Covid-19 is a beta-coronavirus, SARS-CoV-2. Like all coronaviruses, SARS-CoV-2 makes a protease that is required for it to reproduce. Without an active protease, the virus cannot copy itself. To block the protease is to inhibit virus replication altogether—and to put a halt to the disease’s deep creep into the body.
Two prior coronaviruses epidemics, SARS and MERS, triggered an intense hunt for anti-coronavirus drugs. Several researchers discovered chemicals capable of inhibiting the SARS and the MERS proteases, several of which prevent viral replication in the laboratory. Some even inhibit replication in infected laboratory animals. But when SARS was successfully contained, and when MERS proved to be a highly localized epidemic, interest in approving these drugs for human use waned.
Now comes the best news—the discovery of drug candidates that specifically inhibit the SAR-CoV-2 protease. The researchers responsible for the discovery began with a leg up. Previous studies elucidated the precise means by which drugs inhibit the SARS and MERS proteases, right down to the atom-to-atom contacts made between the drug and the protease. Below is a photo of a sculpture by Mara G. Haseltine, SARS Inhibited (2006), that depicts the active site of the SARS protease.

SARS Inhibited (2006) is a bronze sculpture that stands in the central plaza of the science city Biopolis, Singapore. As you walk through and alongside it, you become a protease inhibitor.
MARA G. HASELTINE
The SARS-CoV-2 protease closely resembles that of both the SARS and MERS enzymes, giving the drug developer a great guide. Using this information, the researchers designed two chemicals, designated 11a and 11b, they thought should do the job. Then, they conducted a series of experiments to test their idea, summarized in the series of questions below:
Do 11a and 11b bind to the protease? Yes, they do—and tightly.
Do 11a and 11b prevent the protease from cutting other proteins in test tube experiments? Yes, they do.
Do 11A and B prevent virus replication in the lab? Yes, they do, and at very low concentrations. 11A is more potent 11B, but both work.
Does the drug reach a level predicted to be effective? Both drugs do when injected into mice and dogs. 11a has a longer half-life and greater bioavailability than 11b.
Is the drug toxic? No toxicity was observed in mice and dogs given what are considered to be therapeutic doses of 11a. No toxicity was observed in dogs given very high doses of 11a.
For these reasons, the authors conclude that “11a is a good candidate for further clinical study.”
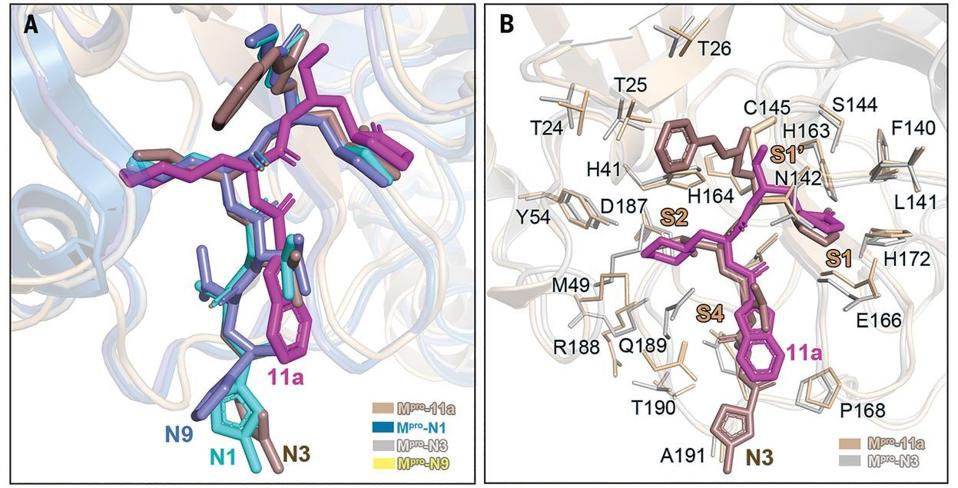
From the study: “(A) Comparison of binding modes of 11a in SARS-CoV-2 Mpro with those of N1, N3, and N9 in SARS-CoV Mpro. SARS-CoV-2 Mpro–11a(wheat; PDB code 6LZE), SARS-CoV Mpro–N1 (sky blue; PDB code 1WOF), SARS-CoV Mpro–N3 (gray; PDB code 2AMQ),
DAI, W., ZHANG, B., JIANG, X. M., SU, H., LI, J., ZHAO, Y., XIE, X., JIN, Z., PENG, J., LIU, F., LI, C., LI, Y., BAI, F., WANG, H., CHENG, X., CEN, X., HU, S., YANG, X., WANG, J., LIU, X., … LIU, H. (2020). STRUCTURE-BASED DESIGN OF ANTIVIRAL DRUG CANDIDATES TARGETING THE SARS-COV-2 MAIN PROTEASE. SCIENCE (NEW YORK, N.Y.), 368(6497), 1331–1335. HTTPS://DOI.ORG/10.1126/SCIENCE.ABB4489
What are the next steps? First, measuring the bioavailability and toxicity of 11a in macaque monkeys.
Then, answering the following questions:
Does 11a reduce the viral load of macaque monkeys infected with several different isolates of SARS-CoV-2, and if so at what dose?
What’s the best dosing regime to assure permanent clearance of SARS-CoV-2 infection in macaque monkeys?
If successful, 11a will then begin phase 1 safety trials in healthy humans. This will be a dose-escalation trial in which increasing doses of the drug are administered to healthy volunteers, most likely in groups of twenty to forty per dose. The bioavailability and half-life of the drug in humans will be measured, along with the analysis of the breakdown products blood and urine. Such a test should be completed within two months. I suspect that they are already in progress, if not already complete.
If the phase 1 trials are successful, the ability of 11a to decrease viral load in volunteers with active SARS-CoV-2 infection will be examined next. Again, small groups of SARS-CoV-2 positive volunteers will be given increasing doses of 11a. To monitor any reduction or drug-related toxicity, viral load will be measured using nasopharyngeal swabs, saliva, bronchoalveolar lavage, and feces. Within days, researchers should know whether or not 11a performs as hoped.
Approving the drug for clinical use can take one of two possible routes. One is based on the ability to decrease viral load. Such was the case for approved anti-HIV drugs, for which reduction in the incidence of AIDS isn’t required. This is in part due to the long delay between infection and disease.
The situation is different for Covid-19. The disease appears with mild symptoms within a week of infection, and with serious symptoms requiring hospitalization within two to three. Rather than a reduction of viral load, approval might be based on a reduction of mild to moderate symptoms (including chest x-rays to measure silent lung infection) or a significant decrease in the number of people who progress from infection to serious disease requiring hospitalization.
Given the urgency of our need, I favor approval based only on the ability of the drug to decrease the viral load by several orders of magnitude in all samples.
The good news is that regardless of the fate of 11a, this research group and many others are hot on the trail of a variety of drugs that will inhibit proteins needed for viral replication. I predict with confidence that by this time next year, cocktails of anti-viral drugs will be approved for use that effectively treat SARS-CoV-2 infections.
I also predict that thereafter, long-acting anti-viral drug formulations will be developed to prevent infection. Such drugs would function as short-acting vaccines to protect our healthcare workers and household members who test SARS-CoV-2 positive.
Lastly, I am confident that also by this time next year, an entirely different approach—use of anti-SARS-CoV-2 monoclonal antibodies—will be effective in tracing and preventing SARS-CoV-2 infections. In any case, help is on the way!
Originally published on Forbes (June 29, 2020)

