A New Immunotherapy For Cancer: PD-L2
(Posted on Tuesday, July 16, 2024)
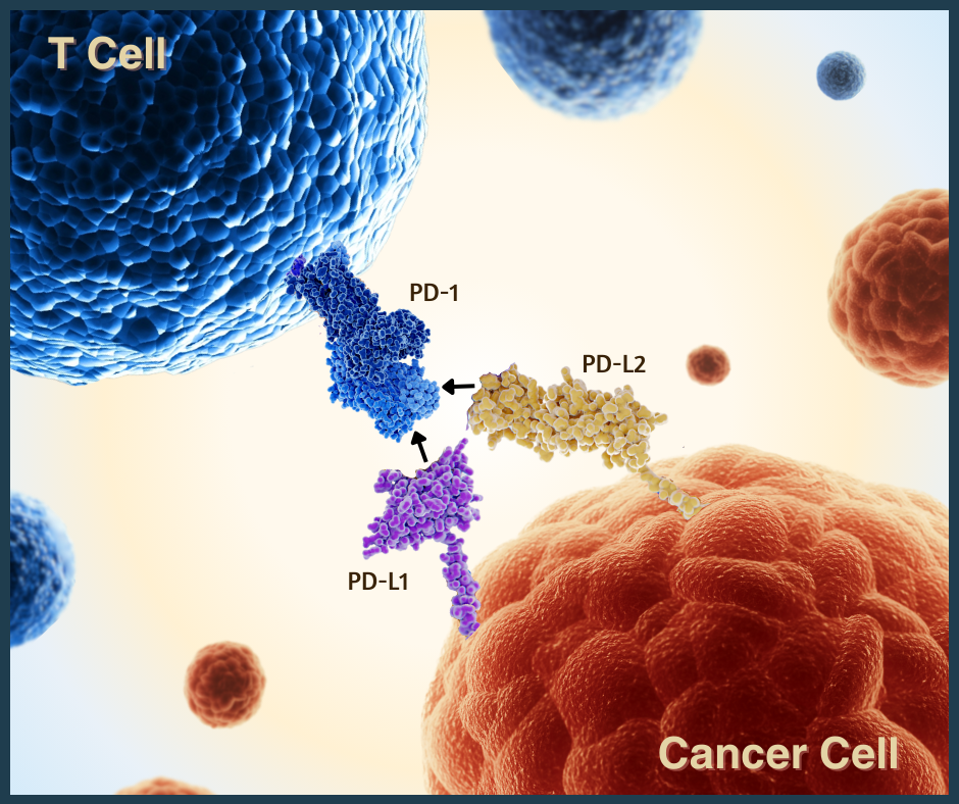
PD-1 checkpoint proteins on T cells (blue) can bind to two different receptors: PD-L1 and PD-L2.
There are many ways to climb a mountain. This saying especially rings true in cancer research, where scientists are probing several avenues to combat tumors. One promising strategy involves checkpoint inhibitors, anticancer drugs that provide encouraging yet sometimes inconsistent results for those with advanced cancers. Some patients respond well to the therapy initially, but resistance can develop and cause the cancer to resurface. There are efforts to refine these treatments—to ensure longer-lasting efficacy and fewer adverse effects.
As part of a series on cancer treatments, this article will delve into a new approach: inhibitors that attack a protein called PD-L2. As the picture shows, this protein interacts with PD-1, an approved anticancer target. Though currently experimental, this mechanism could complement existing PD-1 and PD-L1 checkpoint inhibitors and improve patient outcomes.
How Checkpoint Inhibitors Work
Checkpoint inhibitors are one of many tools to fight cancer. For this treatment, patients receive intravenous infusions filled with antibodies. The antibodies enter the bloodstream and target special molecules found on immune cells called checkpoint proteins. By blocking checkpoint proteins, the therapy unleashes the immune system’s innate ability to fight cancer.
Although the treatment blocks checkpoint proteins, this does not mean immune checkpoints are inherently damaging. In fact, the immune system normally relies on these proteins to slow down overactive immune cells. This prevents immune cells from harming healthy tissues, as seen in autoimmune diseases.
The issue lies with cancer cells. Tumors can express different proteins on their cell surface to help them grow unchecked. This protein expression differs from that of normal cells and even varies from cancer to cancer cell. Some cancer cells use checkpoint proteins to silence immune cells that would otherwise recognize and retaliate against them.
Checkpoint inhibitors release these cancer-fighting immune cells. They bind to checkpoint proteins before cancer and other cells can, freeing the immune cell to activate.
A New Contender: Checkpoint PD-L2
Over the years, researchers have discovered many checkpoint proteins. To date, all US-approved inhibitors target one of three checkpoint proteins: CTLA-4, PD-1 or PD-L1.
Anti-PD-1 and PD-L1 inhibitors are particularly interesting because, despite targeting different molecules, they block the same immune checkpoint pathway. Programmed cell death protein 1 or PD-1 is a checkpoint mainly found on T cells. When it binds to a partner molecule on other cells called PD-L1, or programmed cell death ligand 1, it discourages the T cell from retaliating against tumors. As illustrated in Figure 1, blocking either protein can trigger a strong antitumor response.
Yet, PD-1 possesses another, lesser-known partner—a protein known as PD-L2 or programmed death ligand 2. Independent of PD-L1, this protein tightly binds to PD-1 checkpoints to quiet killer T cell responses. It can also play a role in tumor migration and progression. However, PD-L2 expression is normally more restricted than its counterpart.
PD-L2 is garnering attention as a potential antitumor target. The protein is expressed in multiple cancer types—sometimes more so than PD-L1, sometimes in the absence of PD-L1. Examples include renal cell carcinoma, head and neck squamous cell carcinoma, and cervical cancer. It is also associated with poor patient outcomes, albeit inconsistently. By inhibiting PD-L2, it may be possible to enhance antitumor immune responses, particularly in cancers where PD-L2 expression is prominent.
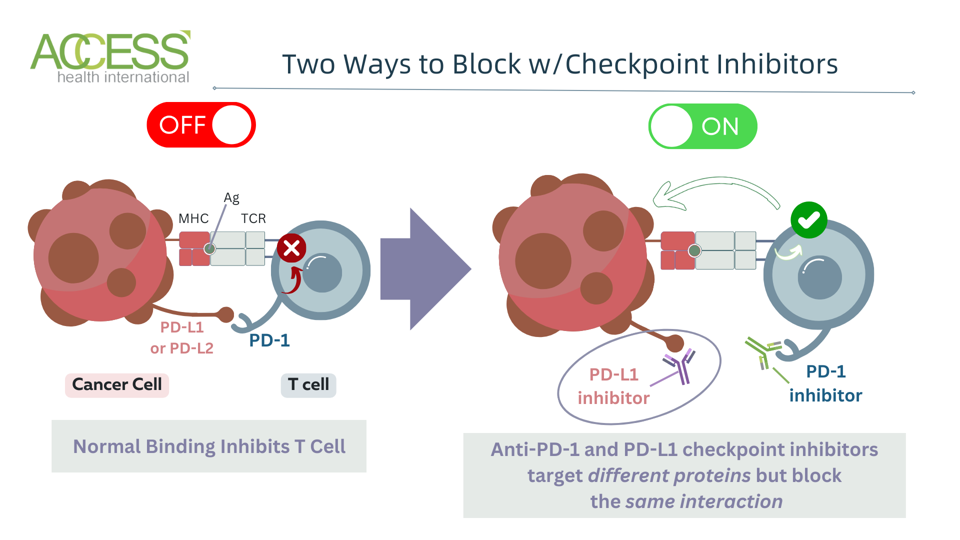
Figure 1: Checkpoint PD-1 (programmed cell death protein) binds to two partner molecules: PD-L1 or PD-L2 (programmed cell death ligands 1 or 2.) While some inhibitors target PD-1 or PD-L1 checkpoints, none currently target PD-L2 alone.
ACCESS HEALTH INTERNATIONAL
Developing Therapeutic Potential
While previously overlooked, PD-L2 interactions play a crucial role in the immune system and should be considered as a therapeutic target alongside other immune checkpoints. Targeting this protein may be necessary to optimize current checkpoint inhibitors, which struggle to suppress certain tumors. Ideally, existing therapies could be altered or combined with other drugs to widen their therapeutic breadth to include more PD-L2 targeting.
How, then, can PD-L2 targeting be improved? This unanswered question has several potential solutions.
One solution is to improve PD-1 inhibitor binding. A preclinical study of ovarian tumors employs a soluble PD-1 inhibitor that binds more strongly to its partner molecules than standard PD-1 inhibitors. The soluble inhibitor successfully blocks both PD-L1 and PD-L2 interactions and reduces ovarian tumors, which are notoriously difficult to treat with checkpoint inhibitors.
Another option is to develop independent anti-PD-L2 inhibitors. They could be given alongside other checkpoint inhibitors to attack several immune pathways or supplement other cancer therapies, such as chemotherapy.
One study demonstrates how such an inhibitor can combat a negative effect of chemotherapy called senescence. Senescence is a normal phenomenon that prevents damaged or cancerous cells from multiplying. However, chemotherapy and other cancer treatments can induce a form of senescence that actually encourages tumor growth. These cancerous, senescent cells resist immune detection using PD-L2 checkpoints. PD-L2 inhibitors could block these checkpoints, leaving the cells susceptible to treatment. In fact, the preclinical experiment demonstrates that while their PD-L2 inhibitor alone does not suppress tumor growth, combined with chemotherapy, it can eliminate tumors with minimal adverse effects.
PD-L2 targeting could also be integrated into novel cancer vaccines. These vaccines contain immune cells, such as dendritic cells or macrophages, with silenced PD-L1 and PD-L2 genes. Since these cells lack checkpoint proteins, the vaccine should stimulate the immune system to recognize and attack tumors without shutting off killer T cells in the process.
Lastly, small-molecule drugs could be engineered to directly block the protein, as one clinical trial investigates various solid tumors and lymphomas. Alternatively, the medicines could target transcription factors and other proteins that indirectly impact PD-L2 expression on cells. Both options could be implemented alongside existing checkpoint inhibitors to boost their effect.
PD-L2 and the Microbiome
An untapped PD-L2 pathway could yield positive results. It was discovered that the protein could interact with another molecule beyond PD-1—a membrane-bound protein known as RMGb, or Repulsive Guidance Molecule b. The interaction between the two molecules regulates immune responses in the lungs and the intestinal microbiome. Encouraging, emerging evidence suggests that blocking this immune pathway may crucially promote microbiome-dependent antitumor responses when tumors resist PD-1 and PD-L1 checkpoint inhibitors. This promising strategy will be explored further in a future installment.
Takeaways
Tumors are contentious adversaries that manipulate the immune system and resist many advanced technologies, including checkpoint inhibitors. Improving PD-L2 targeting in anticancer drugs could give us the edge needed to suppress cancer cells for longer. Its therapeutic viability will continue to unfurl as research reveals more about this protein’s regulatory mechanisms and its synergistic effects with other cancer therapies.

