A Vaccine Candidate Protects Non-Human Primates From SARS-CoV-2 Infection
(Posted on Wednesday, April 29, 2020)
A pre-print in BioRxiv from a Beijing based biotechnology company company, Sinovac, describes protection of macaque monkeys from infection by SARS-CoV-2 by a vaccine candidate. The candidate is a “killed virus” vaccine prepared by inactivating live virus with beta-Propriolactone, a standard procedure used in inactivate other viruses for use as vaccines. Alum, used in many vaccines, was added to the killed virus preparation to elicit a stronger immune response. To my knowledge this is the first published report of protection of non-human primates by a COVID-19 vaccine candidate.
Preparatory studies of the vaccine candidate were conducted in mice. The vaccine candidate administered day 0 and day 7 elicited strong antibody responses that neutralized all eleven strains of SARS-CoV-2 isolated in China and several European countries. The majority of the antibodies were directed against the part of the spike protein, called the receptor binding domain, necessary for virus infection.
Monkeys were injected with two different doses of the vaccine candidate at day 0, day 7 and day 14. All animals produced produced strong antibody responses to viral proteins including the spike protein. A separate group of animals were inoculated with a sham vaccine containing all ingredients of the vaccine except the inactivated virus. A third group was left untreated. Both doses of the vaccine induced strong neutralizing antibody responses by day 21.
All the animals were challenged on day 22 by introducing live virus into the lung. As expected within 3-7 days all the animals inoculated with the sham vaccine as well as the untreated control animals were infected and fell ill. All the animals inoculated with the higher doses showed no signs of infection nor was virus detected in the fluids obtained from the throat, lung or rectum. Transient infection was observed in of animals inoculated with the lower dose as evidenced by isolation of live virus from the fluids in some of the animals.
There has been a concern that an anti-coronavirus vaccine might enhance the disease, as was observed several years ago for a vaccine intended to protect cats from a coronavirus induced diarrhea (a virus unrelated to SARS-CoV-2). This phenomenon is called antibody dependent enhancement (ADE). The authors report that ADE was not observed in any of the vaccinated animals.
These are the hoped for results for candidate vaccine for human trials. Sinovac has already initiated safety trials of the vaccine candidate in 144 volunteers in China. If successful, a trial involving 1,000 volunteers in planned for mid-May to evaluate efficacy of the vaccine. Sinovac has received support from the Chinese government both to conduct the trials and to scale up manufacturing capacity. Sinovac currently produces several other killed virus vaccines for the prevention of other diseases.
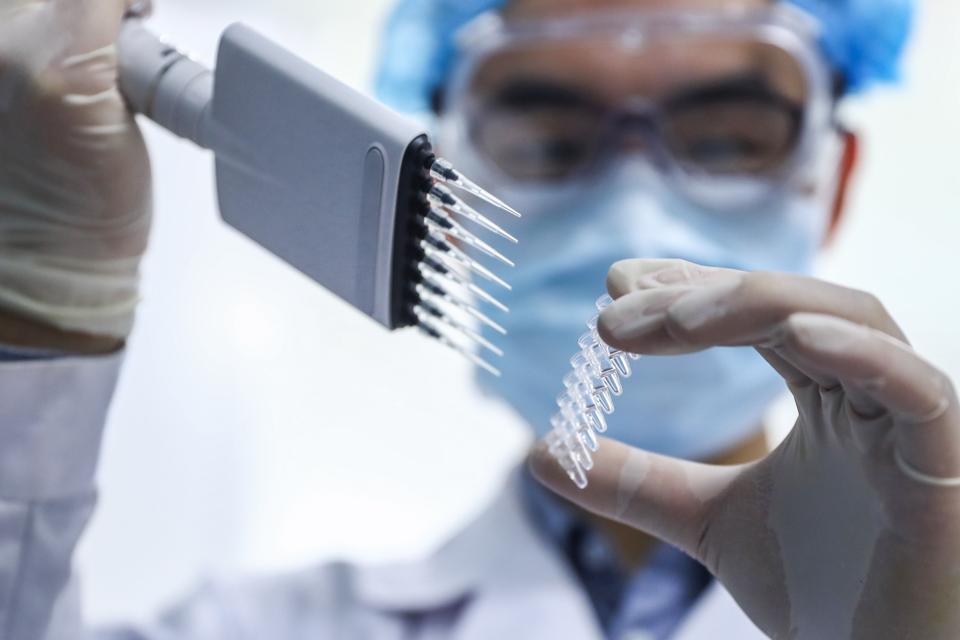
BEIJING, April 11, 2020 — A staff member tests samples of the COVID-19 inactivated vaccine at a vaccine production plant of China National Pharmaceutical Group in Beijing, capital of China, April 11, 2020. China has approved two COVID-19 inactivated
XINHUA NEWS AGENCY/GETTY IMAGES
This is by no means the only vaccine to enter human trials. According to the World Health Organization, as of this week there are seven vaccine candidates in human trials and many more are on the way.
I am personally encouraged by the results presented. The vaccinated monkeys raised exactly the type of antibodies that are likely to be most protective and the most serious feared side effect, ADE, was not observed. The Sinovac vaccine may or may not be the one that eventually brings a halt to the pandemic but we are off to a very promising start.
Note. This manuscript was not peer reviewed prior to posting.
Originally published on Forbes (April 25, 2020)