Altered CAR T Therapy Shrinks Ovarian Tumors In Mice
(Posted on Wednesday, March 22, 2023)
Serous carcinoma is the most common type of ovarian cancer, accounting for approximately 75% of epithelial ovarian cancers.
GETTY
Cancer treatments are rapidly evolving with the advent of cell therapy. Within the last five years alone, CAR T therapy has become widely recognized for its ability to harness the immune cell’s natural abilities to clear cancer and enhance them with genetic modification. Unfortunately, this major achievement is currently restricted to clinical treatment of leukemia and similar blood cancers. Could this standard design be changed to treat solid tumors, too? A study published in The Journal for ImmunoTherapy of Cancer attempts to answer this question in a mouse model of ovarian cancer. The researchers tweak this oft-used design to improve cell signaling and create a CAR T cell which is more capable of shrinking solid tumors than its standard counterparts.
A Unique Challenge: CAR T Therapy & Solid Tumors
Cancer arises when cells malfunction and lose their ability to stop growing. The uncontrollable and detrimental growth of these cells could manifest either liquid tumors or solid tumors.
Liquid tumors refer to cancers which stem from the bone marrow and circulate the blood. Leukemia rests within this group, alongside lymphoma and multiple myeloma. Solid tumors, in contrast, form a mass of cells and constitute around 90% of all adult cancers. For now, CAR T therapy can only be used clinically to treat blood cancers. The reasoning comes down to the cell’s design and the unique nature of solid tumors.
Fundamental CAR T Cell Structure
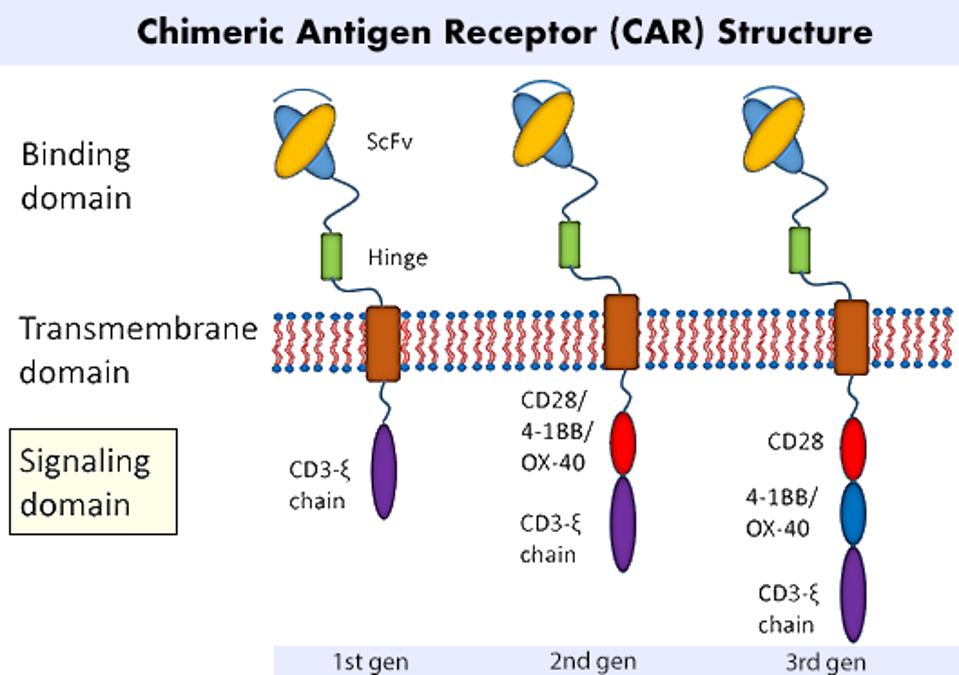
The principle behind Chimeric Antigen Receptor T cell (CAR T) therapy involves extracting a patient’s immune cells and altering them to fight cancer once reinfused. The most basic CAR T cell contains two fundamental components: a cancer-detecting region found on the cell surface, and a signaling region found naturally within the cell (see Figure 1). The synthetic, antibody-like fragment detects any cells which have a particular antigen or biological tag. The signaling machinery releases chemicals to kill the target. This combination of natural and unnatural elements in the cell’s receptor underlines the therapy’s chimeric nature.
The antibody-like fragment (single chain variable fragment, scFV) is programmed to hone in on a single target. This means that CAR T therapy indiscriminately clears cells regardless if they’re healthy or cancerous; all that matters is if a particular tag is detected. This is fine for patients with blood cancers, as the affected cells can be supplemented with additional treatments. This strategy, however, is not effective against solid tumors.
FIGURE 1: A CAR T cell’s most basic form attaches a synthetic cancer-detecting structure onto existing T cell signaling machinery. Variations in this basic model yield first, second and third generation designs, among others. The difference between each generation lies in the number of costimulatory molecules present. Costimulatory molecules CD28 or 4-1BB are commonly used.
LIU ET AL., 2019.
Inhospitable Solid Tumors
Solid tumors present distinct challenges which current iterations of CAR T cells struggle to overcome. First, unlike for liquid tumors, it can be very difficult to pinpoint an antigen target that is unique to solid tumors. Many potential targets overlap with antigens found on normal tissues; unlike with supplemental antibody treatments, it is not possible to replace this tissue if attacked.
Due to the additional tissue barrier, it is physically more difficult for CAR T cells to travel into solid tumors. The T cells need to penetrate the cancerous tissue to be effective. In comparison, liquid tumors circulate the blood and thus are much easier to access.
Lastly, solid tumors possess an extensively inhospitable microenvironment for the T cells. The tumor environment is hypoxic, meaning it lacks oxygen needed for metabolic processes. Additionally, these tumors actively suppress immune responses which CAR T cells need to eliminate the cancer.
Scientists believe that CAR T cell design could potentially meet these challenges—but not without changes first. A team from the Karolinska Institutet in Sweden tests a potential version of CAR T therapy to treat ovarian cancer in mice.
Ovarian Cancer and CAR T Cell Signaling
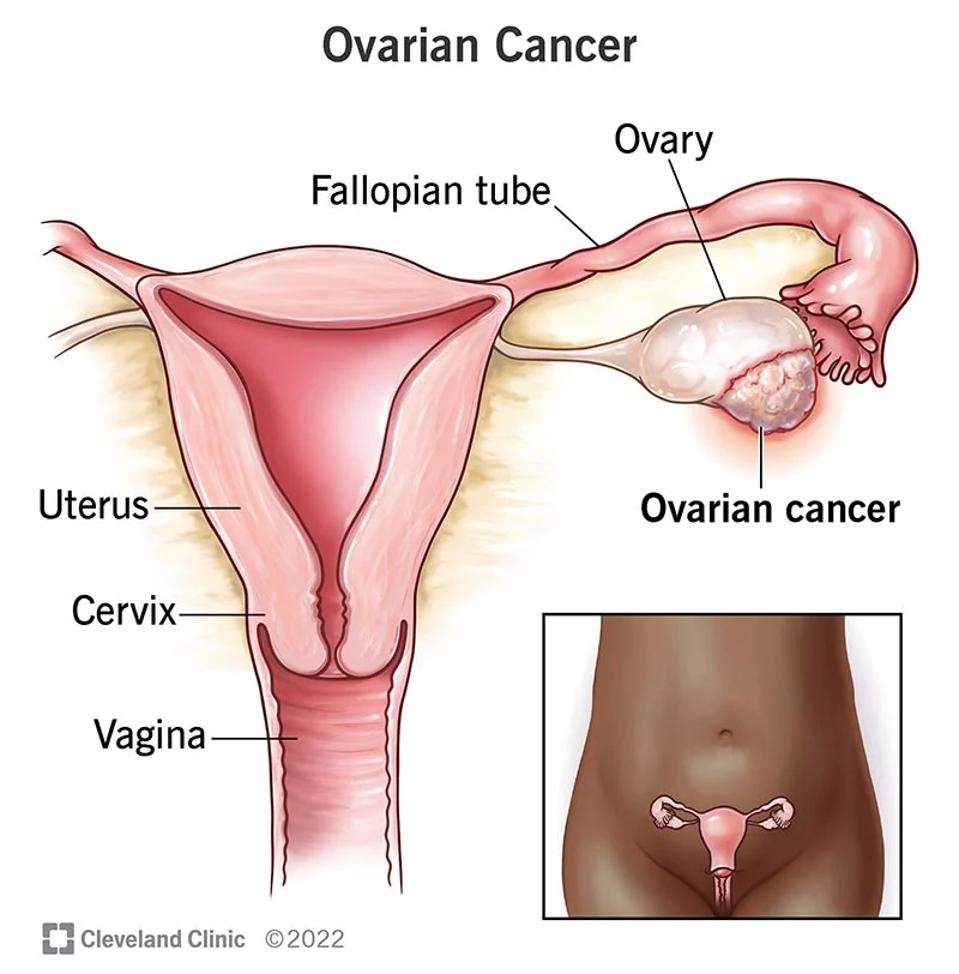
The ovaries are small organs responsible for producing eggs and female hormones. As seen in Figure 2, cancerous tumor masses can form on this pair of organs and disrupt normal functionings. More than 19,000 women will receive a new diagnosis for ovarian cancer this year. The 5 year survival rate hovers around 49%. Most treatments entail surgery, radiation or chemotherapy, but perhaps CAR T therapy could eventually be added to the mix.
In their study, Schoutrop, et al. created three types of CAR T cells for ovarian cancer. Figure 3 illustrates the differences in design.
All three of the therapies targeted human mesothelin, a surface protein which is overexpressed in many ovarian tumors. They all contained a costimulatory molecule used in keeping with second generation design. All second generation CAR T cells contain a version of this molecule, usually either CD28 or 4-1BB (see Figure 1). It releases a secondary signal which bolsters the cell’s performance or survival, strengthening the main signal as a wifi booster would. Notably, the six CAR T therapies on the market for blood cancers also use a second generation design, but target different antigens: either CD19 or BCMA.
Mutating CD3ζ Molecule
The important distinction lies not in the costimulatory molecules, but with activation of the main signal. For one of the CAR T cells, the team mutated DNA sequences in the CD3ζ molecule of the chimeric antigen receptor (see Figure 3). They specifically reduced the number of ITAMs (short for immunoreceptor tyrosine-based activation motifs) sequences from three to two, and juxtaposed its performance with other second generation CAR T cells.
While these motifs are certainly involved in T cell signaling, it is unknown whether all motifs are needed to send a clear signal. Based on background research, the team predicted that the mutation would finetune the cell’s activation signaling cascade and improve cell persistence.
FIGURE 2: The ovaries are female reproductive glands which produce eggs. For people with ovarian cancer, the cells of one or both of ovaries begin to multiply uncontrollably and can cause tumors.
CLEVELAND CLINIC
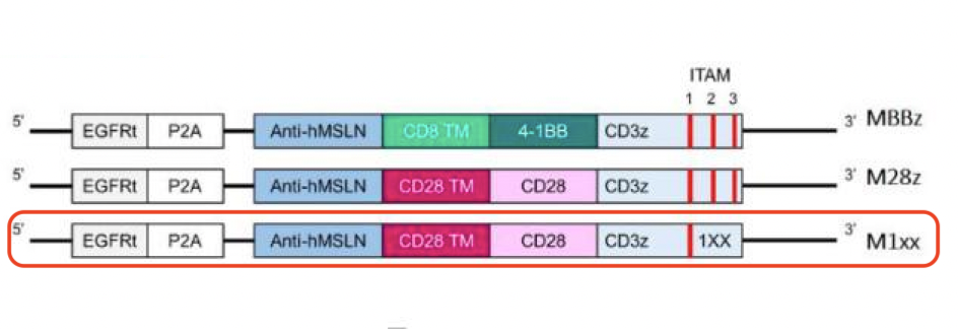
FIGURE 3: The researchers constructed second generation CAR T cells with an anti-mesothelin antibody fragment, a CD28 costimulatory molecule and a mutated CD3ζ signaling domain (in red). The mutation removes two DNA sequences—immunoreceptor tyrosine-based activation motifs (ITAMs) 2 and 3. To judge the resulting changes, the mutated CAR T cell is compared to similar second generation CAR T cells with either a 4-1BB or CD28 costimulatory domain (MBBz and M28z respectively).
SCHOUTROP ET AL., 2023.
Study Results
The researchers tested their three CAR T cell designs in cell cultures and mouse models of ovarian cancer. The cell culture experiments entailed repeatedly exposing ovarian cancer cells to one of the three CAR T cells in test tubes (and a control). All three mesothelin-related T cells displayed multifunctionality and similar tumor-killing potential.
To test the synthetic cells in vivo, mice received a transplantation of ovarian tumor cells and received a CAR T cell treatment 21 days after. The team then tracked the tumor burden for the control and all three cell treatments using bioluminescent monitoring. Although all three CAR T therapies decreased tumor burden, the mutated CAR T treatment outperformed the rest. The tumor size comparison seen in Figure 3 and 4 clearly demonstrates this significant antitumor response. In fact, more than 50% of mice treated with the mutated T cells had undetectable signs of tumor.
The team did a parallel analysis to further analyze how the mutations in CD3ζ influence tumor burden. The CAR T cell which shared the same costimulatory molecule but lacked the CD3ζ mutations reduced tumor growth in mice injected with ovarian cancer cells. However, it did not surpass the survival spurred by the CD3ζ mutated T cells.
Based on these observations, the researchers concluded that removing the two DNA motifs did indeed calibrate the CAR T cell’s activation. This, in turn, improved the CAR T cell’s persistence and antitumor responses—two qualities which are needed to confront the unique challenges presented by solid tumors.

FIGURE 4: The researchers monitored tumor burden through weekly bioluminescence monitoring. In red, the mutated CD3ζ CAR T cell displayed significantly less tumor burden than its un-mutated counterparts.
SCHOUTROP ET AL., 2023.
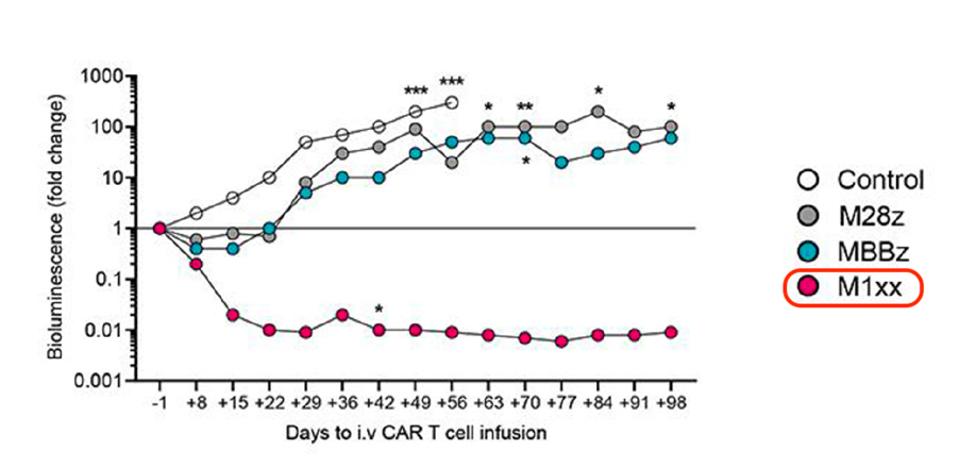
FIGURE 5: The resulting tumor burden differed significantly between interventions. Of the group, the CAR T cell infusion with the mutated CD3ζ chain demonstrated the least tumor growth in the mice.
SCHOUTROP ET AL., 2023.
Looking Forward
The researchers in this study adjusted CAR T cell signaling and achieved notable success in shrinking ovarian tumors in mice. They show that, despite the success CAR T therapy has already achieved, there is still room yet to expand its potential applications. In particular, a focus on streamlining CD3ζ may be an important key in efforts to treat solid tumors with CAR T therapy.