How SARS-CoV-2 Evades And Suppresses The Immune System (Part Two)
(Posted on Friday, August 13, 2021)
This is the second article in a 15-part series called “Relevance of Immune Suppression by SARS-CoV-2 to Understanding and Controlling the Covid-19 Pandemic,” which will explore an underappreciated but highly significant aspect of SARS-CoV-2 replication. The ability of SARS-CoV-2 to delay, evade, and suppress the immune system has myriad implications for drugs, vaccines, and other aspects of our pandemic response. The first set of pieces in this series are intended for a general audience; the second set, for the medical community; and the third and final set, for biomedical researchers looking for a deeper understanding of variants, how they’re generated, and what we might do to control them. Read part one.

Vintage undercover spy stealing files in a filing cabinet late at night, security, data theft and crime concept
GETTY
Asymptomatic infection
Like Covid-19, SARS emerged from long-incubating animal reservoirs. Why then did only Covid-19 spread far and wide? The reason is very likely the relative lethality between the two. People infected by SARS and MERS knew almost immediately, so severe were the symptoms. This made it easy for health officials to identify and control almost every case.
Not so for Covid-19. Though SARS-CoV-2 is certainly capable of causing severe illness and death, neither is the primary outcome. This virus is characterized first and foremost by its propensity for asymptomatic infection, which causes more than 50 percent of new infections. Proliferation, not pathogenesis, is its selective advantage. SARS-CoV-2 has evolved mechanisms that allow it to evade and suppress innate immunity, many of which kick in early, allowing the virus enough time to enter, proliferate, and exit before the immune system can respond.
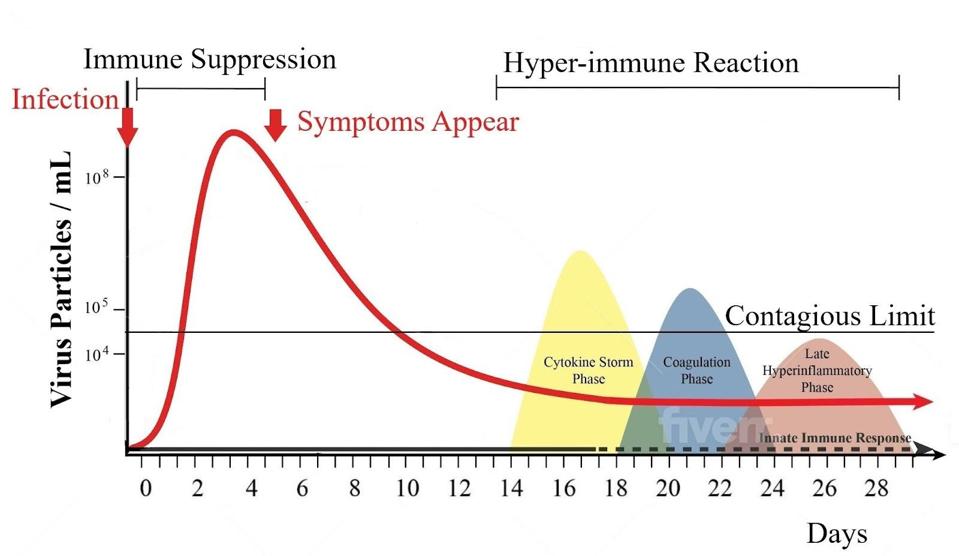
Figure 1: Virus particles per milliliter in nasal secretions over time. Symptoms appear one or two days after the peak of virus load.
ACCESS HEALTH INTERNATIONALMethods of immunosuppression
The moment SARS-CoV-2 infects a cell, it begins to counter both the innate and adaptive immune response. The primary target is interferon, the protein that protects the infected cell and alerts surrounding cells to the presence of an invading pathogen. Studies show that Covid-19 patients are at higher risk of severe illness and death when their ability to produce a robust interferon response is compromised.
Upon infection, SARS-CoV-2 initiates a multilayered program that assures that little to no interferon is made and that which is made cannot reach its target. Several viral proteins specifically block the production and function of interferon and interferon-stimulated genes. This is no coincidence—it is the interferons alpha and beta that induce both the innate and adaptive immune responses.
Once inside, the virus creates a special organelle that conceals both viral proteins and the replicating structure, including double-stranded RNA, from several cellular watchdogs that would normally trigger the alarm system. By building this special compartment, the double-membrane vesicle, the virus avoids inducing the production of interferon and interferon-responsive proteins.
In addition to concealing its replicative activities, SARS-CoV-2 disables cellular protein synthesis. In doing so, it not only clears the way for the preferential synthesis of its proteins, but also prevents the synthesis of interferons.
The virus disguises its messenger RNAs as if they were cellular, eluding yet another defensive tripwire. The first viral protein made obstructs ribosomal messenger RNA entry, preventing translation of all messenger RNAs while opening the gate to the messages of SARS-CoV-2. Viral proteins also block nuclear export and splicing of newly made messenger RNAs, including interferon messenger RNAs.
Another pair of viral proteins compromise export of all cellular proteins, ensuring that any warning signals made by an infected cell will not alert neighboring cells to the trouble nearby. An array of viral proteins prevents the initiation of messenger RNA synthesis of both interferon and interferon-induced genes. Another viral protein stymies interferon and interferon-induced proteins by inhibiting signaling protein transport and entry into the nucleus.
In other words, SARS-CoV-2 mounts a full-court press against the initiation of both the innate and adaptive immune response. This covert strategy fends off the immune reactions long enough for the virus to enter and exit before a naive or memory response kicks in.
The virus has several additional strategies for re-entering previously exposed populations. As a free particle, it remains vulnerable to high concentrations of neutralizing antibodies directed for the most part at the surface glycoprotein, the spike trimer. Mutations in the spike that retain the ability to infect, yet elude the neutralizing antibodies of the previously infected, allow endless cycles of infection of adult immunocompetent populations. Such is the natural history of cold-causing coronaviruses, and such would likely be the story of SARS-CoV-2 without our intervention. Mutations may also increase the avidity of the spike protein for the receptor or stabilize the spike, increasing infectivity. Still other mutations may increase the half-life of the free virus particle. Variants may also arise that replicate more rapidly dramatically increasing the concentration of exhaled particles.
All of the above is only a brief overview of the many immunological tricks SARS-CoV-2 has in its repertoire. Next up in this series I will examine how they evade our first line of defense: innate immunity.
Originally published on Forbes (August 13, 2021)

