New Study Predicts Immune Protection May Vary For Different Covid-19 Vaccines
(Posted on Friday, March 26, 2021)
TURIN, ITALY – MARCH 24: An Italian military nurse prepares the vaccine COVID 19 by AstraZeneca at the Covid-19 vaccine hub of Esercito Italiano at Juventus Allianz Stadium on March 24, 2021 in Turin, Italy. The test swab area at the drive in
GETTY IMAGES
After more than a year of lockdowns, isolation, and great loss of human life, the slow but steady rollout of Covid-19 vaccines has given us all a much-needed glimmer of hope. As of March 22, the total number of doses administered worldwide is nearing 500 million, with millions more to come as the eligibility pool expands from high priority groups to the broader population.
But for all the progress being made, much remains unknown about how protected people really are—and how long that protection will last—once they’re vaccinated, especially now that more infectious and immunologically challenging variants of SARS-CoV-2 are on the loose. Though it will be some time before we have long-term clinical data on vaccinees that can answer these questions definitively, a new preprint study has taken the first step by using predictive modeling techniques to estimate the strength and length of immune protection conferred by seven different vaccines over time. The results suggest that the more protective a vaccine is immediately following immunization, the longer protection will last. More than that, however, they imply we’ll have to retool our vaccine strategies so they better address the issue of waning immunity against Covid-19.
To predict the trajectory of immune protection for each vaccine, the researchers premised their model on the fundamental assumption that high levels of neutralizing antibodies correlate with immune protection—an observation that has come up numerous times in previous research on reinfection in recovered Covid-19 patients and vaccine safety and efficacy. Using data on Pfizer-BioNTech, Moderna, Sputnik-V, Bharat Biotech, Johnson & Johnson, AstraZeneca, and SinoPharm vaccines, they were able to plot how the neutralizing antibodies generated by each one fell over a theoretical timeline of 250 days. The researchers then compared these trajectories to those of recovered Covid-19 patients who had natural immunity vis-a-vis data on convalescent sera, in addition to modeling how lower antibody titers might fare against new SARS-CoV-2 variants. They also used convalescent sera as a baseline for normalizing the vaccine data, which in its original state came from a diverse array of assays and was difficult to collate as a result.
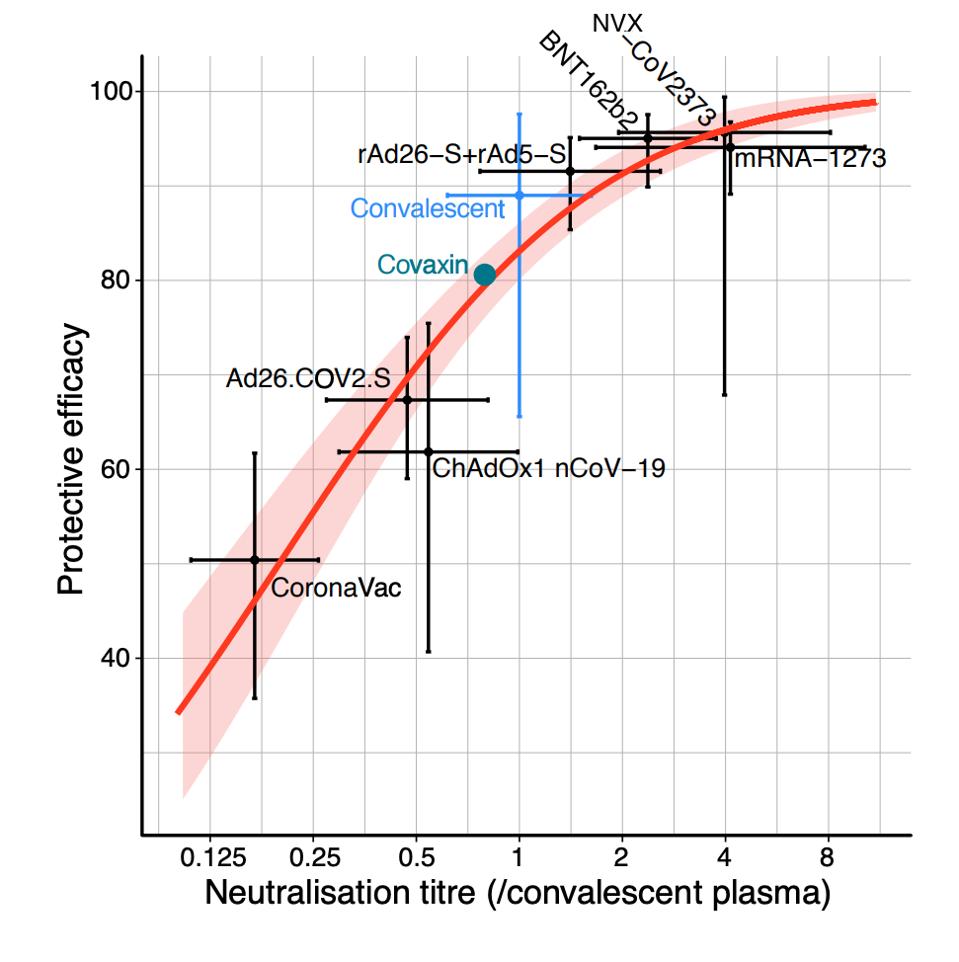
Figure 1. Relationship between neutralization level and protection from SARS-CoV-2 infection.
HTTPS://WWW.MEDRXIV.ORG/CONTENT/10.1101/2021.03.09.21252641V1.FULL.PDF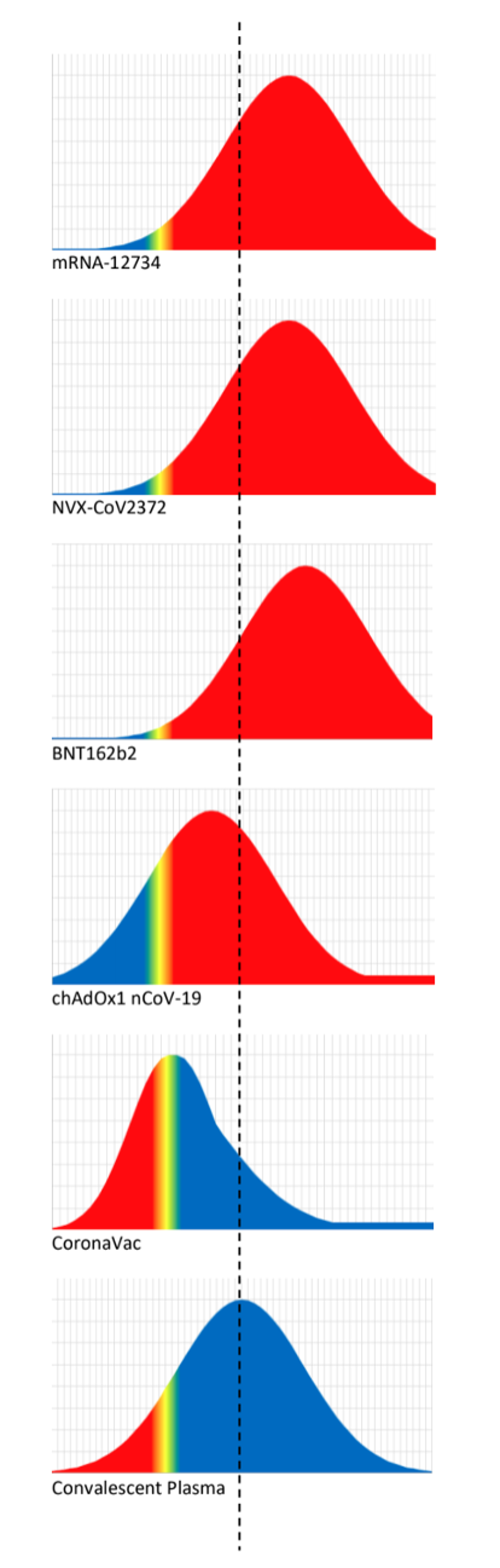
Figure 2. Schematic illustration of the logistic approach to identifying the protective neutralisation level.
HTTPS://WWW.MEDRXIV.ORG/CONTENT/10.1101/2021.03.09.21252641V1.FULL.PDFUltimately, the researchers’ hope is that the evidence-based modeling study, in their words, “will assist in developing vaccine strategies to control the future trajectory of the pandemic.” One consideration such strategies will have to take into account is that protection from disease isn’t the same as protection from infection. When the researchers cross-analyzed the neutralization titers produced by each vaccine and plugged the numbers into their model, they found that protection from severe Covid-19 held steady, but protection from infection by SARS-CoV-2 declined significantly. By how much, according to their model, depended on the initial strength of the neutralizing antibody response.
The second consideration, which I’ve already written about at length in my viral variation series for Forbes, is that the new SARS-CoV-2 variants are proving to be more difficult to neutralize than the original wild-type virus that emerged out of Wuhan, China. Several studies have already documented how the efficacy of the first generation of Covid-19 vaccines—even at their most potent, in the days following immunization—falters against the B.1.351 variant in particular, remaining protective but not as much as before. The predictive model echoed this, showing that the variants pose a greater threat to vaccines with a lower initial efficacy against the wild-type virus. If the initial efficacy of a vaccine is around 70 percent, for example, and the neutralizing titers of the vaccine are reduced five-fold due to a new variant, the researchers predict efficacy will fall to just 25 percent.
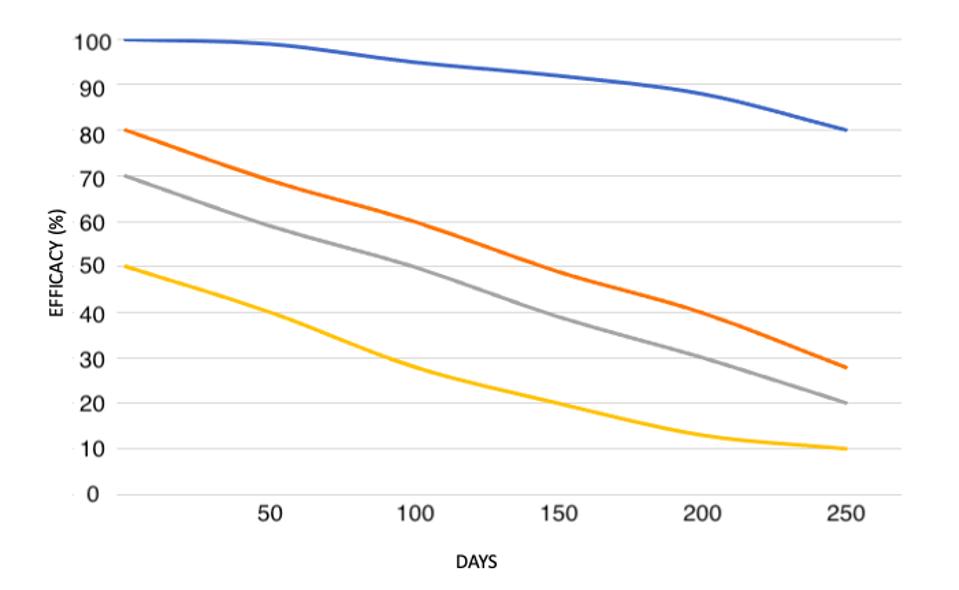
Figure 3. “Predicting the effects of declining neutralisation titre. Assuming the observed relationship between neutralisation level and protection is consistent over time, we estimate the decline in efficacy for vaccines starting with different
HTTPS://WWW.MEDRXIV.ORG/CONTENT/10.1101/2021.03.09.21252641V1.FULL.PDF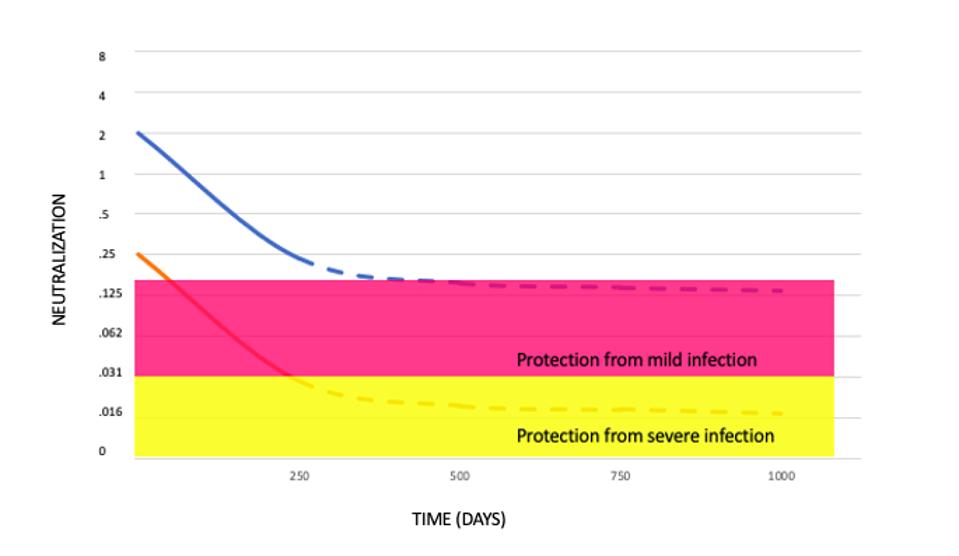
Figure 4. Extrapolating the decay of neutralisation titres over time.
HTTPS://WWW.MEDRXIV.ORG/CONTENT/10.1101/2021.03.09.21252641V1.FULL.PDF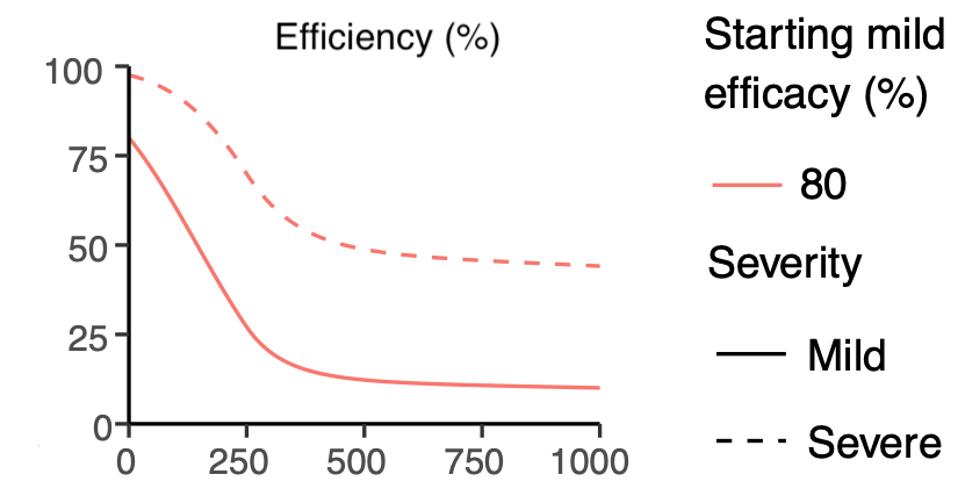
Figure 5. Extrapolating the trajectory of protection for a vaccine with an initial efficacy of 80%.
HTTPS://WWW.MEDRXIV.ORG/CONTENT/10.1101/2021.03.09.21252641V1.FULL.PDFThis brings up a third and final consideration—that not all vaccines are created equal. While the correlation between initial efficacy and duration of protection is consistent across all the vaccines, their individual neutralizing antibody titers varied widely. The Pfizer and Moderna mRNA vaccines performed the strongest, with an initial efficacy of 95 percent that didn’t drop to 50 percent until around day 200. By contrast, the Johnson & Johnson and AstraZeneca adenovirus vaccines, which had an initial efficacy of 67 and 62 percent respectively, reached the 50 percent mark around day 50. At the far end of the spectrum was the Sinopharm vaccine, which had an initial efficacy of 50 percent in the first place and, within the same amount of time, offered next to no protection at all. If this predictive model is indeed an accurate reflection of reality, that means those who receive the Sinopharm vaccine will have the same level of protection immediately after immunization as those who received a Pfizer or Moderna vaccine 200 days prior. The Pfizer and Moderna vaccines also had immunization trajectories similar to those of recovered Covid-19 patients, while the Johnson & Johnson, AstraZeneca, and Sinopharm vaccines fared worse.
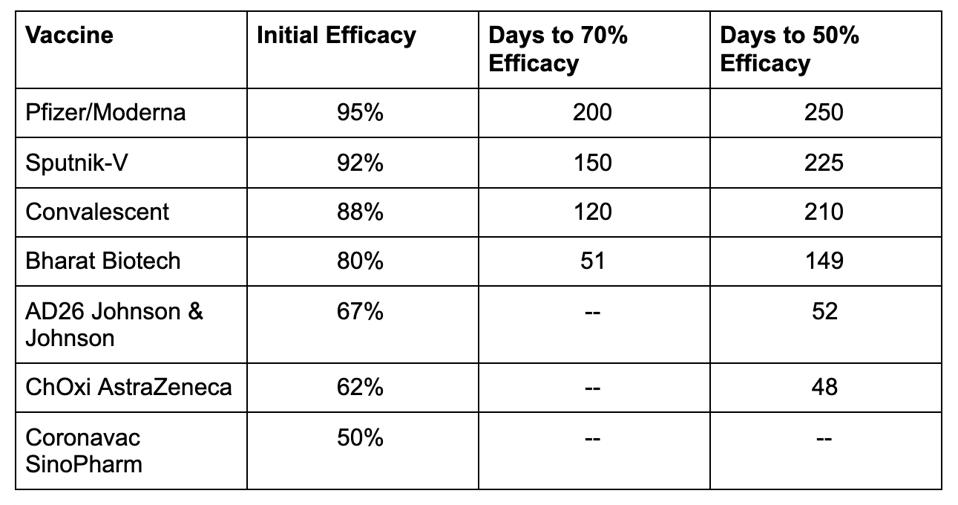
Table 1. Comparison of reductions in efficacy over time based on data from the modeling study.
HTTPS://WWW.MEDRXIV.ORG/CONTENT/10.1101/2021.03.09.21252641V1.FULL.PDFThis isn’t to say that any of the above vaccines should be ruled out as ineffective—quite the contrary. Nor does it necessarily contradict current public health messaging, which dictates that if we’re given the opportunity to get vaccinated against Covid-19, we should take it, no matter the type. Ultimately, the researchers acknowledge, this study is predictive, not prescriptive, and it comes inbuilt with many caveats, among them the lack of standardization across neutralization assays and the potential role of T cells in providing immune protection. The biggest caveat by far is that these calculations aren’t based on real measurements, but hypothetical averages taken from available data on convalescent sera. In actuality, as a recent study on Singapore Covid-19 patients makes clear, the duration of the neutralizing antibody response varies from individual to individual, with a very small fraction experiencing very long persistence and another fraction, no antibodies at all.
But the modeling study still serves as a dire warning—to vaccine developers, policymakers, and anyone who thinks this initial wave of vaccinations will be enough to protect us from the virus for the remainder of 2021. If we don’t reconfigure our vaccine strategies to address the discrepancies between vaccines and their reduced potency over time and against the variants—especially that of the adenovirus vaccines—we risk leaving some vaccinees more vulnerable than others for no reason other than negligence and complacency. For as hopeful as it is that many of us will be vaccinated by the end of this year, if we don’t look to the future, we risk ending up back where we started: isolated, unprotected, and wondering why our public health leaders didn’t act sooner.
Originally published on Forbes (March 25, 2021)

