Fighting Cancer: A Basic Guide to Checkpoint Inhibitors
(Posted on Monday, May 6, 2024)
This article is part of an ongoing series on novel cancer advances, including immunotherapies such as CAR T therapy and checkpoint inhibitors. Future installments will continue to demystify checkpoint inhibitors and explore the latest research expanding the field.
In March 2011, a new cancer advance burst into the spotlight. This drug, known as a checkpoint inhibitor, stood out from chemotherapy and other standard cancer treatments. Rather than directly targeting cancer cells, this immunotherapy bolsters the immune system’s innate ability to fight tumors. Initial clinical trials demonstrated that the therapy can improve overall survival and risk of death for patients with one of the deadliest forms of skin cancer, unresectable or metastatic melanoma.
Today, checkpoint inhibitor research branches far beyond melanoma. Scientists are probing to uncover more about the therapy, especially when administered alongside other cancer treatments. Though the journey to understanding checkpoint inhibitors may be overwhelming, this article can guide you through the first steps. Subsequent articles will delve further into specific checkpoint inhibitors and other recent discoveries.
Checkpoint inhibitors (ICIs) are an antibody-based cancer drug. These infusions contain antibodies that target particular proteins called immune checkpoints.
Typically, the immune system relies on checkpoint proteins to turn off overactive white blood T cells. This mechanism is essential to protecting the body, preventing excitable T cells from harming healthy tissues. However, cancer cells use these checkpoints to their own benefit. By binding to these checkpoints, cancer cells shut off T cells that would otherwise turn against them, leaving them to multiply unrestrained.
Inhibitor drugs release the biological restraints on these T cells. Most inhibitors are designed to bind to T cell checkpoint proteins before a cancer cell can; some inhibitors bind to the checkpoint ligand on the cancer cell instead. Either way, these interactions prevent the cancer cell from silencing the T cell. T cells are then free to activate and eliminate the tumors.
Checkpoint inhibitors were first approved for advanced skin cancer. Now, these drugs can treat over 25 types of solid tumors. The list includes:
- Bladder cancer
- Breast cancer
- Colon and rectal cancer
- Kidney cancer
- Liver cancer
- Non-small lung cell cancer
- Melanoma
- Pancreatic cancer
Checkpoint inhibitors can also treat Hodgkin and non-Hodgkin lymphoma, two types of blood cancers. However, the FDA has not approved them to treat other blood cancers, such as leukemia or myeloma—although this could change with ongoing research. Another recent immunotherapy, Chimeric Antigen Receptor T Cell Therapy (CAR T therapy), has demonstrated more promising results in this arena. Table 1 provides a comprehensive list of cancers treated by specific checkpoint inhibitors.
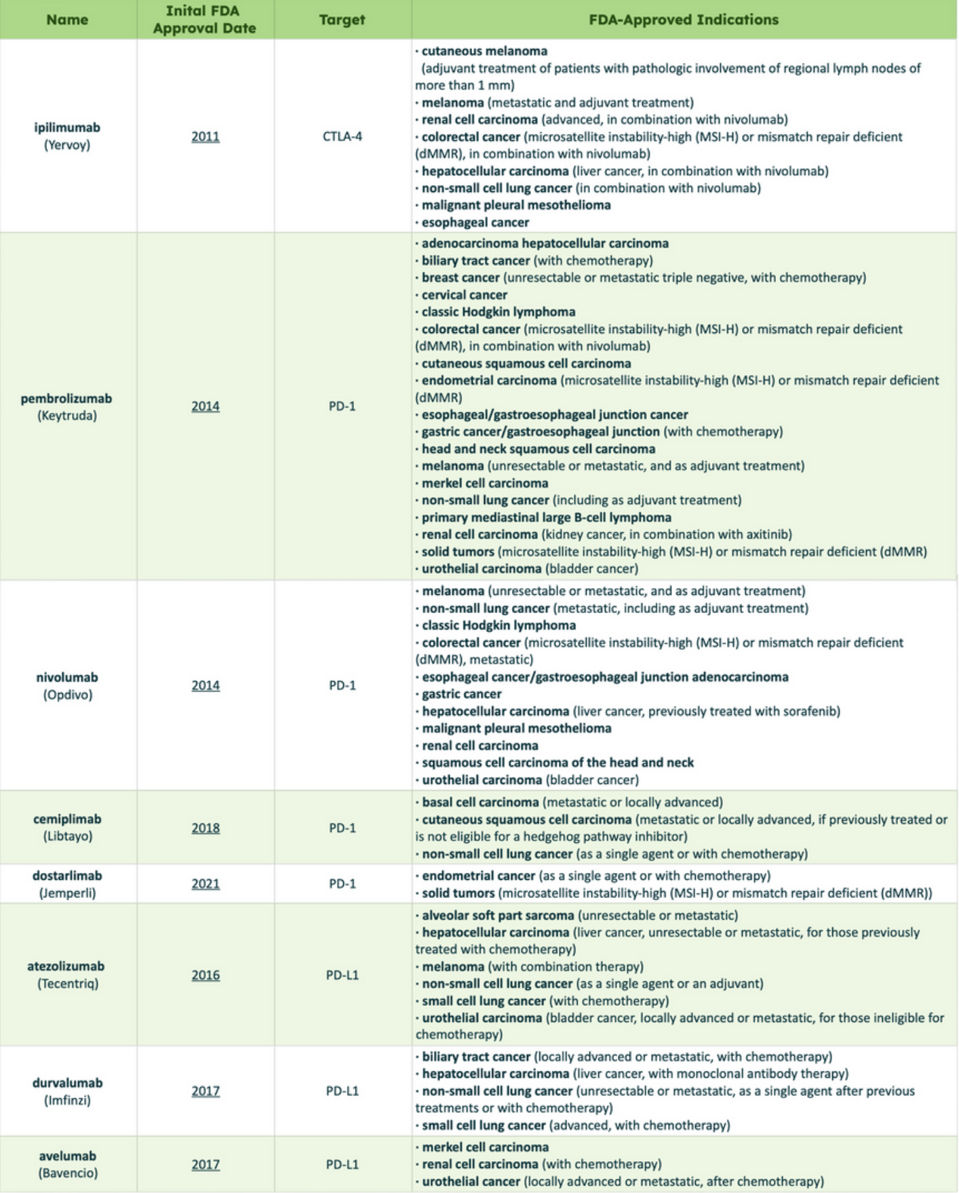
TABLE 1: List of checkpoint inhibitors currently approved by the FDA. Definitions: Adjuvant, treatment before surgery; Unresectable, cancer that cannot be removed entirely with surgery; Metastatic, cancer that has spread.
ACCESS HEALTH INTERNATIONAL
Checkpoint inhibitors are increasingly being given as a first treatment, especially for people with advanced melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (a type of kidney cancer), or Hodgkin lymphoma who are ineligible for a bone marrow transplant. For other illnesses, including bladder cancer or head and neck cancer, checkpoint inhibitors are considered after other standard treatment options prove ineffective.
Inhibitors can also be administered before surgery or alongside other cancer treatments, such as chemotherapy, radiation and CAR T therapy. Combination treatments synergistically target tumors from distinct angles. For example, chemotherapy and radiation directly damage and kill cancer cells, while the inhibitor encourages immune cells to retaliate. Checkpoint inhibition can allow for less invasive surgery or eliminate the need for surgery in some cases.
Ultimately, the decision to turn to checkpoint inhibitors will depend on each person’s baseline health, what treatment options they have tried, and the projected efficacy of checkpoint inhibitors for their disease.
Exactly how effective are checkpoint inhibitors at treating cancer? This can be a difficult question to answer. Checkpoint inhibitors do not cure; rather, they slow or reduce tumor progression to varying degrees. Its efficacy is influenced by several factors, including what treatments a person has already received and the type of cancer at hand. Results can also differ if the infusion is given with other checkpoint inhibitors or cancer treatments.
People with Hodgkin’s lymphoma, melanoma, or solid tumors with specific genetic characteristics (Microsatellite Instability-High, MI-H) tend to respond more readily to checkpoint inhibitor therapy. In one clinical trial, around 87% of participants with Hodgkin’s lymphoma responded to nivolumab, a checkpoint inhibitor by Bristol Myers Squibb. In melanoma, inhibitors can reduce tumor size in around 45-60% of patients with advanced melanoma, a significant improvement over historical response rates of less than 10%. A combination of two inhibitors with different checkpoint targets, CLTA-4 and PD-1, can further improve outcomes, with five-year overall survival rates exceeding 50% in some studies.
Response rates for other solid tumors range from 15% to 30%. However, it is critical to note that many patients do not respond to checkpoint inhibitor therapy.
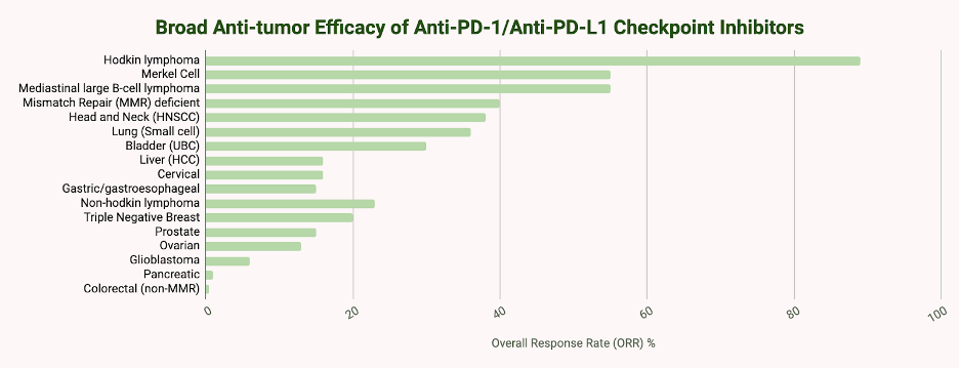
TABLE 2: Overall response rates to anti-PD-1/anti-PD-L1 checkpoint inhibitors by cancer type.
ACCESS HEALTH INTERNATIONAL
Checkpoint inhibitors are delivered into the arm through an intravenous (IV) line. The number of infusions needed varies depending on the specific inhibitor drug. They can be administered once or twice a month for up to a year or more.
A blood test is required before each session. These tests establish a baseline and reference point to monitor for any adverse effects during treatment. Other than that, no other pretreatment is needed. The actual session lasts anywhere between 30 and 60 minutes. Afterward, medical staff will monitor the patient for any unwanted reactions.
Checkpoint inhibitors release a surge of immune responses from the body. This mechanism is vital for attacking cancer but can also encourage the immune system to attack normal, healthy cells. As a result, people who take checkpoint inhibitors may experience immune-related adverse effects.
Immune-related symptoms can manifest in several different organs in the body. The skin can develop rashes, itching, or lose patches of color. The colon, lungs and liver can become chronically inflamed. In rarer cases, inflammation can impact the brain. Inhibitor drugs can also trigger the onset of diabetes or a worsening of pre-existing type II diabetes for a minority of patients (less than 2%).
Clinicians carefully monitor patients before and after each infusion. They may temporarily suspend treatment and implement corticosteroids or antibiotics to manage symptoms.
Transplant recipients or people with underlying autoimmune conditions should carefully consider whether checkpoint inhibitors are suitable for them. Since these inhibitors stir up the immune system, the treatment could worsen pre-existing conditions. Some studies suggest that symptoms are generally manageable, but each healthcare provider will determine the final call on a case-by-case basis.
Notably, these immune-related reactions are usually less toxic than those caused by chemotherapy and radiation, which target cancer cells directly; hallmarks of chemo or radiation therapy, such as alopecia or lowered blood cell counts, are not typical. However, enhanced toxicity may also occur if checkpoint inhibitors are combined with these other treatments.
Checkpoint inhibitors represent a revolutionary advancement in cancer care, offering a promising alternative to traditional treatments such as chemotherapy and radiation. These medications have shown remarkable efficacy by unleashing the body’s immune system to target and destroy cancer cells, particularly in treating advanced melanoma and other solid tumors. However, as with any powerful tool, checkpoint inhibitors possess potential risks and complexities. Furthering this exploration of checkpoint inhibitors, future articles in this series will delve deeper into the intricacies of combining them with other therapies and ongoing research efforts to optimize their effectiveness. Stay tuned for further insights into this evolving frontier of cancer treatment.
Read the original article on Forbes.

