How 1301B7 Could Contribute To Advanced Vaccine Development
(Posted on Friday, June 7, 2024)

This article was originally published on Forbes on 6/7/2024.
In the ongoing Omicron era, a new COVID-19 antibody could renew optimism for urgently required SARS-CoV-2 monoclonal therapies. Over the last three years, various versions of SARS-CoV-2 have mutated to bypass the neutralizing effects of monoclonal antibodies. Whenever new treatments received approval from the FDA, new variants emerged that could avoid these treatments, creating a game of cat and mouse between the virus and researchers looking for new antibody treatments.
A new antibody, 1301B7, was developed by extracting convalescent sera from an individual infected with an earlier strain of Omicron, as described by Dr. Michael Piepenbrink and colleagues at the University of Alabama. The antibody exhibits potent neutralizing activity against multiple SARS-CoV-2 variants, including the latest Omicron subvariants XBB.1.5 and JN.1, and against the sister virus SARS-CoV-1. What follows are the key findings for the promising monoclonal treatment.
The greatest challenge facing antibody developers is the constant mutation of key targetted amino acids. The receptor binding domain and N-terminal domain of the spike protein are common binding sites for antibodies and are common regions of mutation in variants.
1301B7 engages in multiple hydrogen bonds and salt bridges with the receptor-binding domain. The antibody’s heavy chain contacts several critical residues found in Omicron variants, including those involved in ACE2 binding (e.g., R403, Y453, H505). Though the mutation in N417K results in a 20-fold decrease in affinity, 1301B7 maintains its binding to the mutated receptor-binding domain.
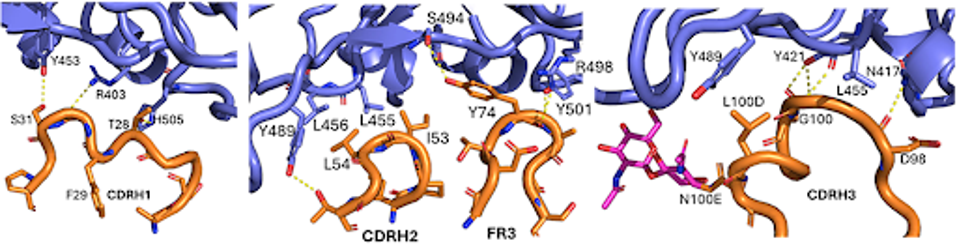
FIGURE 1: Cryo-EM depiction of the antibody (orange) contacting several crucial receptor-binding domain (blue) sites.
A significant contrast between this antibody and previous ones is its distinct binding mechanism, as shown by the cryo-EM structure of 1301B7 attached to the SARS-CoV-2 spike protein. The unique feature of 1301B7 is that it binds solely through its heavy chain complementarity-determining regions without any direct involvement from the light chain.

FIGURE 2: Diagram depicting the difference between heavy and light chains.
In transgenic mice expressing human ACE2, the XBB.1.5 or JN.1 subvariants of the Omicron variant, when treated preventatively with 1301B7 at doses of 2 mg/kg or 20 mg/kg, showed a significant decrease in viral loads in the nasal turbinates and lungs compared to the isotype control.
At four days after infection, mice given 20 mg/kg of 1301B7 showed no signs of virus in the lungs when exposed to XBB.1.5. Only 1 out of 4 mice had any virus in the lungs when exposed to JN.1. These findings illustrate the solid preventive effect of 1301B7 against the most recent SARS-CoV-2 Omicron subvariants.
The authors propose that 1301B7’s wide range of specificity comes from its strong binding and the ability of its complementarity-determining regions to adapt to mutational variations in the receptor-binding domain. This is backed by the finding that 1301B7 utilizes a VH1-69 heavy chain, which has been demonstrated to be produced in response to diverse viruses, such as influenza, hepatitis, HIV-1, and SARS-CoV-2.
The research emphasizes 1301B7’s promise as a strong contender for creating advanced vaccines and treatments for SARS-CoV-2 and its changing versions. Its distinct structural characteristics offer additional enhancement and refinement possibilities using protein—and glycan-focused approaches.
However, several other relevant aspects need to be addressed, which could be pertinent.
1301B7 showed extensive neutralization activity against the tested variants; however, the study did not investigate whether particular mutations in the receptor-binding domain could lead to resistance to 1301B7 binding and neutralization. It would be valuable to comprehend the range of mutations affecting the effectiveness of 1301B7.
The research concentrated on the preventive application of 1301B7 in mice. Its capability as a treatment for active SARS-CoV-2 infections was not assessed. Further research would help evaluate its effectiveness as a treatment in appropriate animal models.
Although monoclonal antibodies are typically well-tolerated, the study did not offer information on 1301B7’s immunogenicity, potential off-target binding, or safety profiles. A thorough evaluation of clinical development would be necessary for these aspects. Even though the study thoroughly described the structural and functional features of 1301B7, further investigation is needed to grasp its potential for clinical application. This includes assessing antibody resistance, therapeutic effectiveness, potential for modification, combination treatments, and safety characteristics.
Perhaps most critically, the authors did not explore the possible combined impacts of pairing 1301B7 with other neutralizing antibodies, antivirals, or current COVID-19 treatments. Assessing these combinations can improve the overall effectiveness and range of protection. Combining multiple antibodies into a combination treatment creates a multiplicated safety net when a contact point is mutated in a later variant. Installing multiple antibody protection levels ensures a more robust long-term protection against immune evasive mutation.
While 1301B7 demonstrates much promise, we have described many promising monoclonal antibodies over the past four years but failed to clear the final hurdles. Ultimately, combining such an antibody with another that targets a more conserved region of the spike or elsewhere in the virus could ensure a more stable long-term treatment, but until then, 1301B7 remains experimental.

