Synthetic Gene Circuit To Hone CAR T Therapy
(Posted on Tuesday, January 4, 2022)

Airplane cockpit control panel
GETTY
Here we describe a new way to control CAR T therapy through synthetic gene circuits. For more on controlling CAR T therapy, read our article on the use of antibody switches to control CAR T cell activation. Previous installments also discuss the fundamentals of CAR T and its applications for B cell cancers, multiple myeloma, lupus, multiple sclerosis and the heart, as well as new research on combination CRISPR/CAR T therapy.
While CAR T therapy represents one of the most impressive innovations in cancer care, the treatment can cause autoimmune-like side effects. Many have contended with the dilemma of maximizing the therapy’s benefits while minimizing its side effects. A recent advancement published in Science shows great promise in improving control of CAR T therapy with the hope to eventually benefit patients.
Challenges of CAR T therapy
Chimeric Antigen Receptor T cell therapy relies on engineering synthetic receptors onto a patient’s immune cells to recognize and eliminate a programmed target. The treatment has proven effective in targeting antigens, or biological tags, found on the surface of some blood cancer cells. Although successful in this regard, the modified T cells cannot be controlled once injected back into the body. This factor, coupled with other obstacles such its inability to target more than one antigen at once, leaves space to improve the treatment.
Possible Solutions
There are several budding research initiatives seeking possible solutions. One previously discussed method of controlling CAR T therapy involves creating CAR T cells which bind to antibody switches. Researchers found that a single infusion of antibody switches could help mitigate the worst of CAR T therapy’s toxic side effects. Although this method could potentially fight solid tumors by altering the antibody target, it has yet to be tested in animal or human models.
A recent study from the scientists at Boston University offers an alternative and versatile platform which could transform CAR T therapy implementation. The team turns to synthetic gene circuits for answers and receives encouraging results.
A New Alternative: Synthetic Gene Circuits
In a gene circuit, the process of turning an input into a desired output can be controlled through the use of a synthetic regulator (see Figure 1). The synthetic regular can, in theory, tailor a cell’s gene expression to produce a desired result. The researchers in this study designed such a circuit using synthetic proteins and small molecule switches.
To establish the circuit, the team relied on synthetic versions of proteins called transcription factors. Transcription factors can recognize certain DNA motifs and help convert that DNA into RNA, genetic instructions for protein synthesis. They leverage synthetic zinc fingers (SynZiFTR) in particular due to its compact size and human origin; Figure 2 illustrates the structure. Both factors together allow the protein to move efficiently in human cells while minimizing unwanted side effects. Additionally, several zinc fingers can be joined together to create a structure capable of recognizing potentially unique human DNA sequences in the genome, as seen in Figure 3.
This circuit must be controlled using a gene switch. The team experimented with three clinically approved small molecule inducers to accomplish this task. The unique combination of zinc fingers and small molecule inducers allowed genes to be turned “on” with the introduction of the inducer, and turned “off” with its removal.

FIGURE 1: A synthetic gene circuit can alter a network of input signals to produce a custom response. Using this underlying base mechanism, CAR T therapy could be controlled through the use of synthetic zinc fingers to produce a more precise result.
LI ET AL.
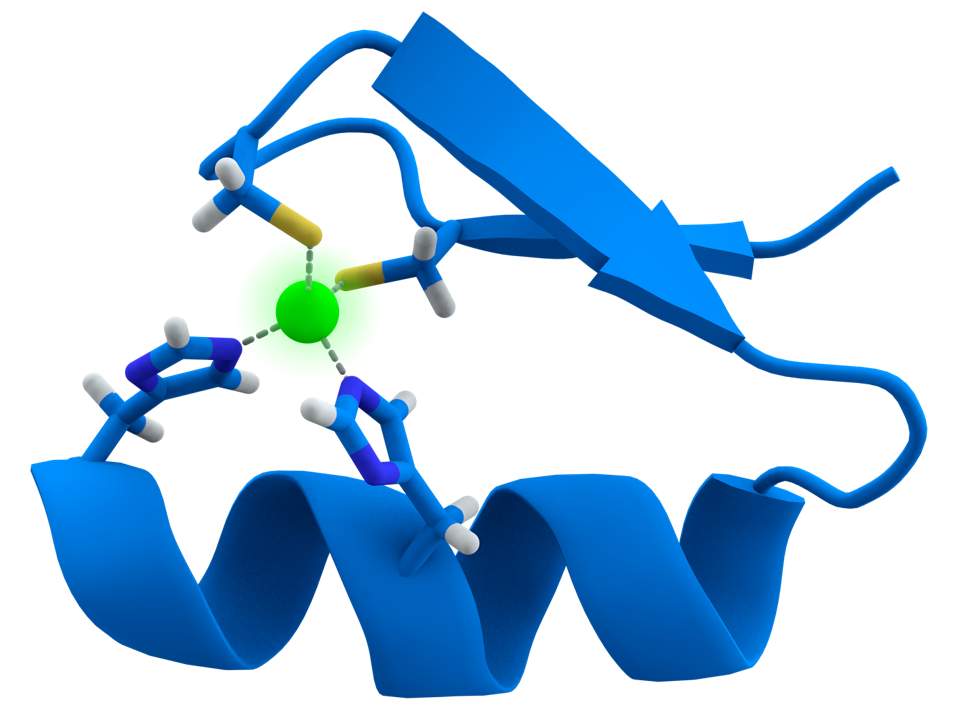
FIGURE 2: Representation of the Cys2His2 zinc finger motif. The zinc ion is represented in green.
WIKIPEDIA
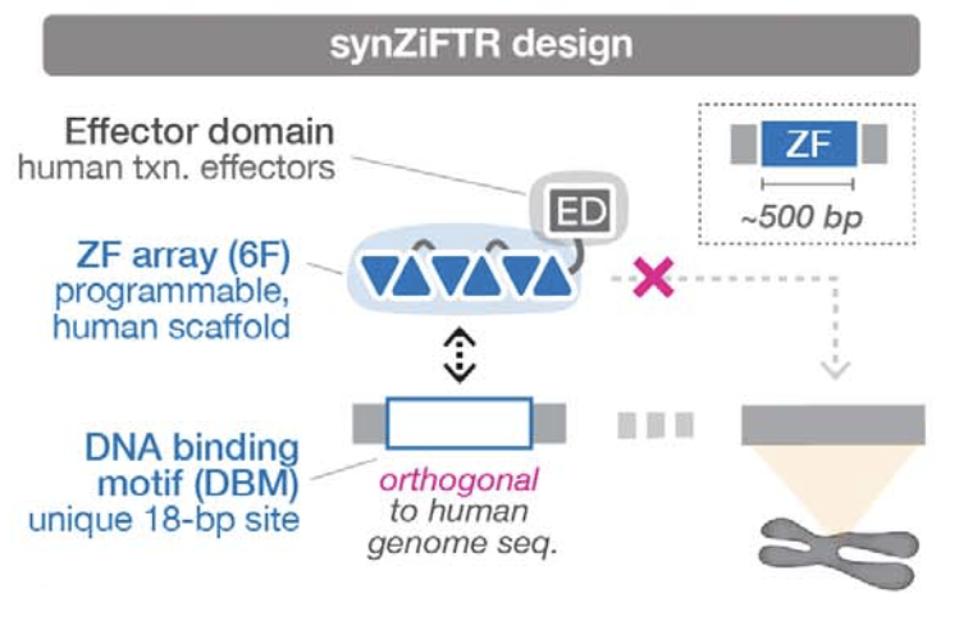
FIGURE 3: Zinc fingers can be modularly arranged with unique DNA binding motifs to modulate transcription in a manner that does not impede other native functions.
LI ET AL.
Combining Synthetic Gene Circuits with CAR T Therapy
How do these synthetic gene circuits fare when used on CAR T cells in vitro and in vivo? The team investigated this question in several stages using cell and animal models.
The researchers first tested the efficacy of a gene circuit which controlled the expression of chimeric antigen receptors. They found that in a xenograft liquid tumor model, the engineered receptor’s expression could indeed be controlled in a drug-dependent manner. The treatment could be reinforced using the same stimuli and secondary infusion of chimeric antigen receptors with a different antigen target, demonstrating the adaptable premise of the platform.
The platform produced similar results when tested on blood tumor models in mice, as well. The mice treated with drug inducer or a drug-increasing cocktail could clear the tumor, while those without had high tumor burden. This phase illustrated how gene circuits can be influenced in a drug-dependent manner in living creatures.
One of the most fascinating findings of this study is the creation of a dual-switch gene circuit. Researchers crafted a gene circuit which impacted cytokines known to influence cellular proliferation. This cytokine circuit could successfully prime dual-switch CAR T cells in mice with leukemia; a secondary signal to encourage CAR expression sparked their anti-tumor activity. The sequential and synergistic effect of the dual-switch circuit can be seen in Figure 4. This duo reduced tumor burden in mice more successfully than untreated CAR T cells or CAR T cells induced with anti-tumor stimuli only.
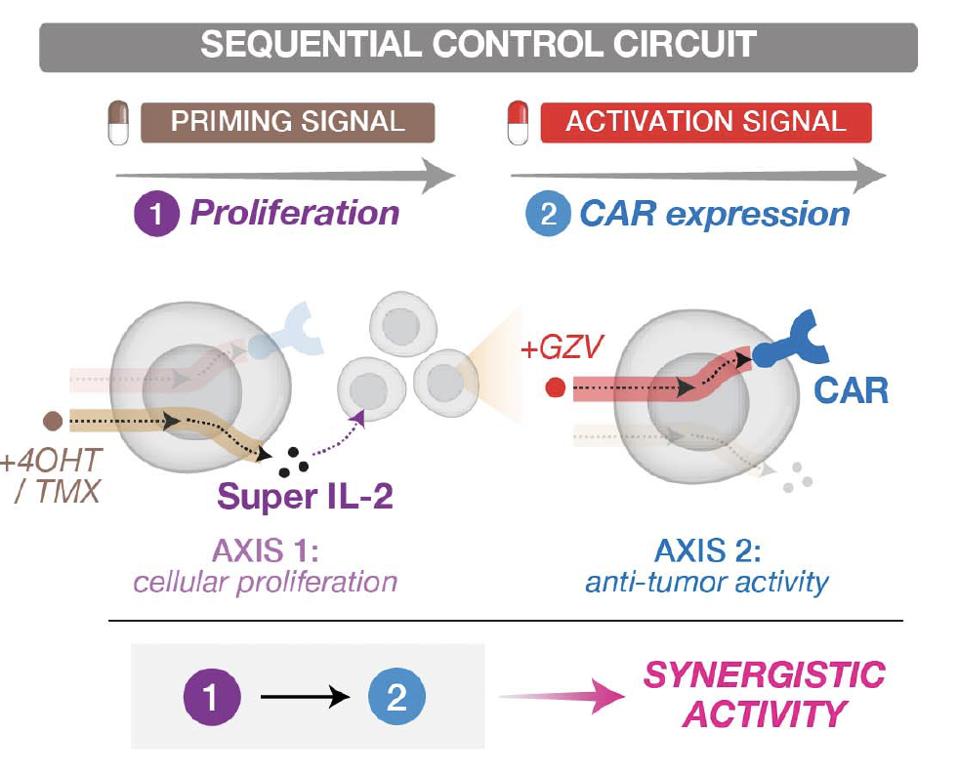
FIGURE 4: Dual-switch gene circuit creates a sequential and synergistic effect by triggering and encouraging cellular proliferation before activating T cell anti-tumor activity.
LI ET AL.
Looking Forward
The researchers here show how synthetic gene circuits can feasibly improve CAR T cell proliferation and anti-tumor activity in animal models. This promising platform could prove clinically viable once translated to humans. However, the implications of this study stretch beyond the rapidly growing field of CAR T therapy. The underlying mechanism could be customized to improve other gene and cell therapies, or combined with other other powerful technologies such as CRISPR-Cas9 to achieve more bespoke results.

