A Drop of Hope for Dry Eyes
(Posted on Sunday, July 16, 2023)
Originally published on Forbes on 7/11/2023
Dry eye disease is a condition that afflicts millions of people worldwide, causing constant discomfort and affecting their quality of life. One of the key culprits behind this condition is meibomian gland dysfunction, which hinders eye lubrication and moisture by preventing the normal secretion of oil from the meibomian glands in the eyelids.
Despite the prevalence of dry eye disease and meibomian gland dysfunction, it remains a mystery as to what exactly causes this condition and how we can address it effectively. However, recent research has made significant strides in understanding the underlying mechanisms of the disease, offering a ray of hope for those suffering from it.
What are Dry Eye Disease and Meibomian Gland Dysfunction?
Dry eye disease is a chronic, multifactorial condition that affects the eye’s surface. It’s a common issue among people of all ages and gender worldwide. Typically, dry eye disease is a result of meibomian gland dysfunction.
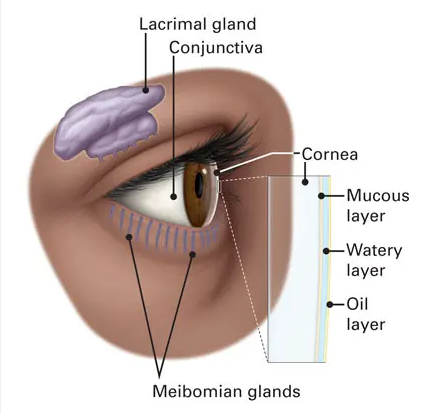
The International Workshop on Meibomian Gland Dysfunction defines this dysfunction as a chronic abnormality of the glands characterized by blockages and changes in secretion. This leads to tear film alterations, inflammation, and other eye-related issues.
Meibomian gland dysfunction manifests in various forms, each with distinct characteristics. The low-delivery state can be obstructive or hyposecretory, while the high-delivery state presents another scenario. These conditions have underlying causes ranging from hyperkeratinization to hormonal fluctuations. Moreover, the dysfunction can also be classified based on the quantity and quality of meibum, which leads to foam formation in the tear film.
Age-related meibomian gland dysfunction adds another layer to the mix. Cell signaling and lipid synthesis changes can occur as we age, contributing to gland atrophy. Reactive oxygen species may also play a role.
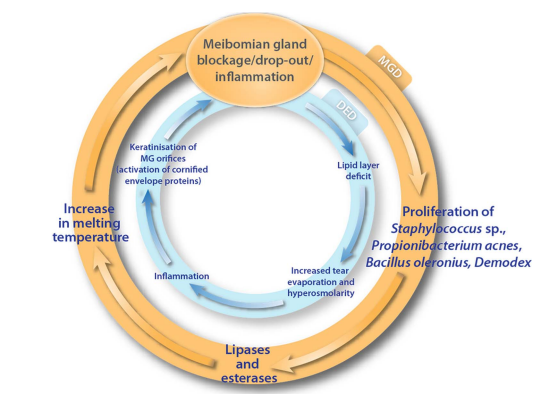
The Vicious Cycle of Dry Eyes
Dry eye disease is like a self-sufficient cycle, causing tear film break-up, drying, inflammation, and more. The condition persists in a vicious cycle even after removing or reducing the initial cause. This happens when the compromised eye surface is continuously exposed to environmental challenges. Understanding the mechanisms of meibomian gland dysfunction and dry eye disease can help us develop more targeted treatments.
One of the entry points into this relentless cycle is the dysfunction of the meibomian gland. When the meibomian gland experiences dropout, blockage, or inflammation, it triggers bacterial growth and spread. These bacteria produce lipases and esterases, increasing the meibum’s viscosity and melting temperature. Consequently, the meibum secretion onto the tear film’s surface is reduced. This defines the self-sustaining circle of Meibomian gland dysfunction.
Insufficient meibum leads to a decrease in lipid content within the tear film. This, in turn, causes increased tear evaporation, hyperosmolarity, and inflammation, perpetuating the cycle of dry eye disease.
Doubling Down on Vicious Cycles:
Meibomian Gland Dysfunction-Associated Dry Eye Disease
The concept of a doubled vicious cycle demonstrates how the disease mechanisms of dry eye disease and meibomian gland dysfunction intertwine to create a chronic condition known as meibomian gland dysfunction-associated dry eye disease.
Meibomian gland changes are a gateway to dry eye disease, perpetuating a vicious double cycle of dysfunction. Advanced imaging techniques, such as in vivo confocal microscopy, optical coherence tomography, and keratography, offer valuable insights into the intricate link between dry eye disease and Meibomian gland dysfunction.
The coexistence of dry eye disease and Meibomian gland dysfunction can contribute to the deterioration of the eye’s surface. Research has shown that when the ocular surface is exposed to stress, it triggers the production of cornified envelope precursors by the ocular epithelium.
These precursors then lead to the keratinization of meibomian gland orifices. Consequently, this can result in meibomian gland inflammation, blockage, atrophy, loss of mucin-filled goblet cells, and entrapment of mucin within the remaining goblet cells, all contributing to the development of dry eye disease.
Finding Relief for Dry Eyes
Dry eyes can be a debilitating condition that can significantly impact a person’s quality of life. Fortunately, various treatment options are available to help interrupt the vicious cycle of dry eye disease.
For those suffering from dry eye and blepharitis, implementing proper eyelid hygiene is one of the most critical steps in managing these conditions. Regularly cleaning the lids and lashes can help remove excess oils and debris contributing to ocular irritation. While anti-inflammatory therapies like oral tetracycline derivatives or topical cyclosporine eye drops can effectively reduce inflammation and improve tear production, patients have another essential option.
Enter Avenova, a lid hygiene product that directly tackles one of the root causes of dry eye and blepharitis: the overpopulation of bacteria that live on the lids and lashes. Studies have shown that Avenova kills harmful bacteria and helps neutralize bacterial toxins and enzymes like lipase, which contribute to these lid conditions. This makes Avenova ideal for controlling eyelid conditions like meibomian gland dysfunction-associated dry eye disease.
Moreover, using tear substitutes is another essential aspect of treating dry eye. These drops or ointments are designed to maintain a healthy tear film and alleviate dryness, burning, and irritation symptoms. Some of the newer tear substitutes on the market also possess osmo-protective properties, which help reduce hyperosmolarity–a key factor contributing to dry eye disease.
Overall, it’s clear that a multi-pronged approach that includes proper eyelid hygiene, anti-inflammatory therapies, and tear substitutes is crucial for managing dry eye. Still, there are some other cutting-edge options out there as well, such as intense pulsed light therapy and vector thermal pulsation.
Intense pulsed light therapy or vectored thermal pulsation may be recommended for those with more severe dry eye disease. It uses a noncoherent polychromatic light source with a 500–1200 nm wavelength spectrum. This therapy helps to reduce inflammation of the glands and improve abnormal telangiectatic vessels through the photothermal effect.
Vector thermal pulsation is a therapy for obstructive meibomian gland dysfunction. It enhances tear film stability by improving the flow of meibomian gland secretions. It’s a non-invasive treatment option when traditional approaches like heat and eyelid massage aren’t practical. This therapy clears meibomian gland obstruction using pulsating pressure and heat.
Each eye receives vectored thermal pulsation for 12 minutes using a temperature- and pressure-controlled device. Adverse effects like eyelid pain, conjunctival congestion, and ocular burning may occur but usually resolve in 4 weeks. Vectored thermal pulsation improves symptoms and signs based on tear break-up time, corneal fluorescein staining, and meibomian gland secretion scores.
Putting the Pieces Together to Take on
Dry Eyes and Meibomian Gland Dysfunction
Dry eye disease is a multifactorial condition affecting millions worldwide. Meibomian gland dysfunction is a prevalent cause of dry eyes, characterized by chronic abnormality and blockages in the glands. Understanding the vicious cycle of meibomian gland dysfunction-associated dry eye disease is essential for developing effective treatments.
By understanding the mechanisms of meibomian gland dysfunction and dry eye disease, we can take significant steps toward reducing the prevalence and severity of this chronic condition. We can look forward to more targeted and effective treatments as research continues.

