Teaming Up Two Biotech Winners to Fight Cancer: CRISPR and CAR T
(Posted on Sunday, December 11, 2022)
Here we describe early clinical trial results on combination CRISPR and CAR T therapy, a sequel to an earlier, introductory piece. Other alternative CAR T designs include mRNA vectors to create temporary CAR T cells and the use of antibody switches to control CAR T cell activation. Previous installments also discuss the fundamentals of CAR T and its applications for B cell cancers, multiple myeloma, and lupus.
Tumor cell, CAR-T cell, treatment by immunotherapy with gene encoding CAR. This illustration shows a
BSIP/UNIVERSAL IMAGES GROUP VIA GETTY IMAGES
CAR T therapy can treat blood cancers by inserting new genes into a patient’s own immune cells using viruses. Early clinical trial results present an alternative that forgoes viral gene transfer: CRISPR technology. Such integration of CRISPR gene editing could improve the precision, speed and cost-effectiveness of CAR T cell production. In addition, researchers hope CRISPR will broaden CAR T therapy applications from blood cancers to solid tumors, which the engineered T cells notoriously have failed to target.
Inserting Genes into CAR T Cells
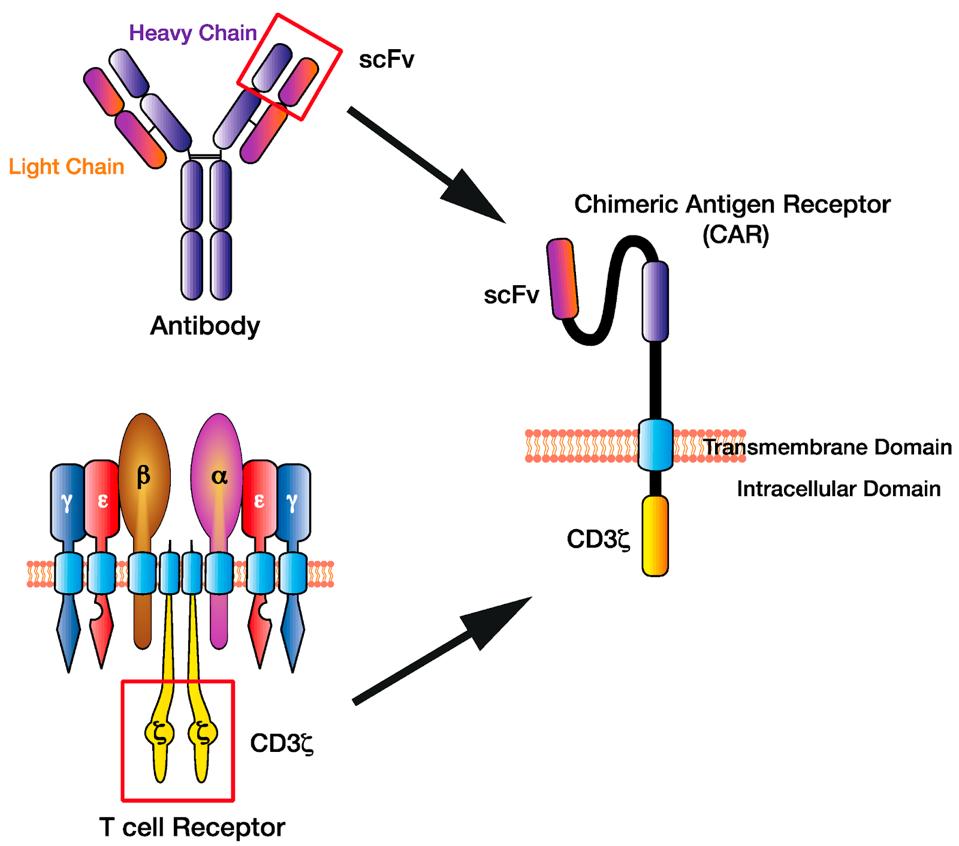
Chimeric Antigen Receptor T cell (CAR T) therapy genetically alters a patient’s T cells to recognize cancer cells and subsequently kill them. This engineered recognition relies on hybrid T cell receptors with antibody components to detect antigens, or biological tags, found on the surface of cancer cells (see Figure 1).
FIGURE 1: Illustration of a chimeric antigen receptor. The structure utilizes an antibody-derived domain to detect specific antigens, all while leveraging a T cell CD3ζ complex for its signal machinery.
HUGHES-PARRY ET AL.
Researchers typically incorporate hybrid receptor genes into a CAR T cell via viral gene insertion. Despite its regard as a staple in cell therapy, retroviral gene transfer comes with several drawbacks. Viral vector manufacturing is expensive and time-consuming. The method lacks precision and could potentially allow an unwanted gene entry. Perhaps most limiting, it cannot be personalized to detect uncommon antigens. For this reason, all approved CAR T therapies in circulation target blood cancers that share a common antigen (usually CD19 or BCMA) rather than solid tumors, which greatly vary in antigen presentation. Standardizing a new means to insert genes would improve the accessibility, efficiency and usage of CAR T therapy.
Innovating with CRISPR Gene Editing
In their Phase I clinical trial, the researchers at PACT Pharma and the University of California, Los Angeles explore the possibility of a different type of CAR T therapy—one that creates a hybrid receptor with CRISPR gene editing. With CRISPR, the team selectively removed native T cell receptor genes and replaced them with new, cancer-fighting alternatives.
The researchers began by searching and isolating a novel T cell receptor from the patient’s own immune system. First, they screened the patients by sequencing DNA from healthy blood samples and tumor biopsies; this step identified mutations which the tumor cells share but cannot be found in normal tissue. Algorithms then predicted which antigens would be present on the tumor.
Next, the team copied the antigens and mixed them with different versions of HLA, a type of molecule needed to present antigens to T cells. This process revealed specific T cells which could react to this particular combination of antigen-HLA. Researchers copied up to three of the highly personalized receptor genes to be integrated into the T cells using CRISPR/Cas9.
Figure 2 illustrates the subsequent process. The CRISPR/Cas9 interface knocked out two T cell receptor genes, TRCα and TRCβ (see Figure 3), and replaced them with three new receptor genes in a single step—decidedly more efficient than sourcing and cultivating retroviruses for gene transfer, as is currently standard in CAR T therapy.
The researchers multiplied the T cells to great numbers. Finally, the patients underwent lymphodepletion chemotherapy before receiving up to three doses of their personalized CRISPR/CAR T cell infusion.

FIGURE 2: An overview of the CAR T therapy using CRISPR technology. Genes for two native T cell receptors, TRAC and TRBC, are removed and replaced with genes for new and personalized T cell receptors.
NATIONAL CANCER INSTITUTE
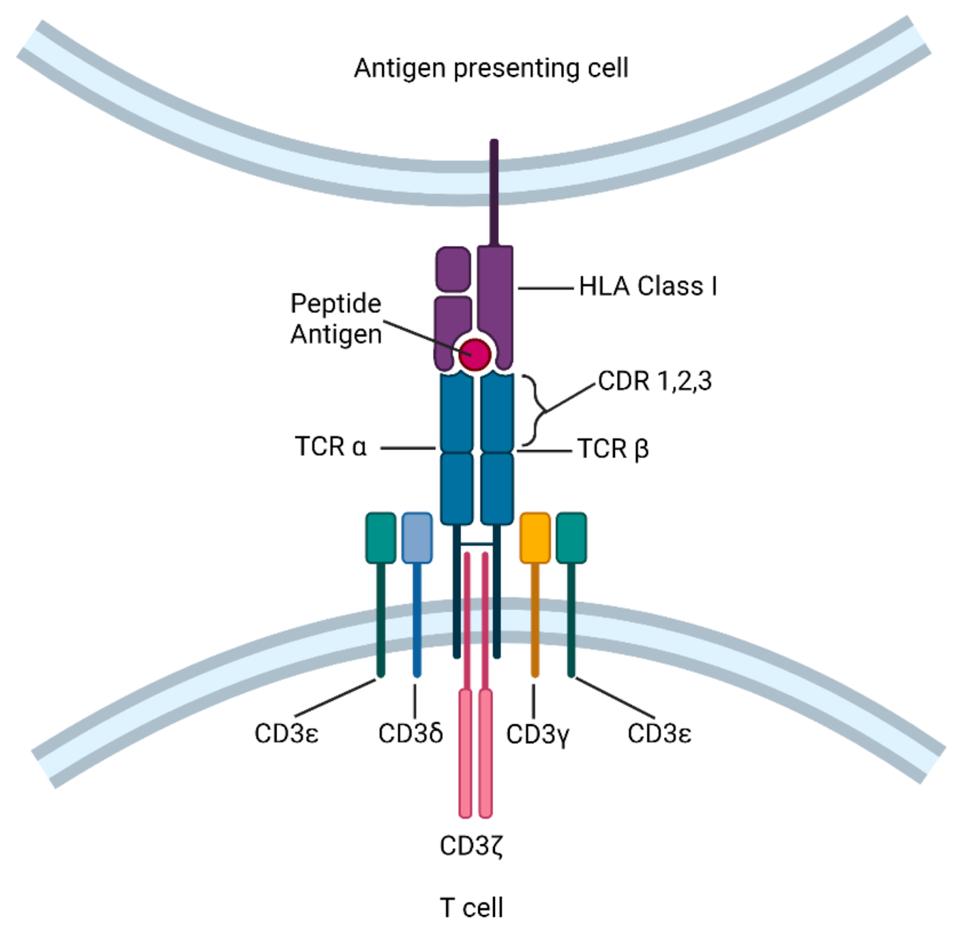
The structure of a T cell receptor (TCR), composed mainly of dimer TRCα and TRCβ and accompanied by a CD3 protein complex. Note the human leukocyte antigen (HLA) molecule present on the antigen presenting cell, denoted in purple; this structure is necessary for the T cell receptor to recognize the antigen peptide.
SUN ET AL.
Results
The researchers assessed the safety and dosage of combination CRISPR/CAR T therapy to treat 16 people with various kinds of solid tumors, including breast and lung tumors. All of the patients experienced drug resistance, and had already received five or more prior lines of therapy.
The CRISPR/CAR T infusion did not prove overly dangerous. All the patients experienced typical side effects of lymphodepleting chemotherapy. One patient in particular experienced mild cytokine release—an expected side effect of CAR T therapy. Another experienced severe encephalitis.
The combination CRISPR/CAR T therapy did not cure any patients. Through biopsies, the team found that the CAR T cells successfully multiplied and traveled into the tumors of eight participants. Four weeks after infusion, five participants had stable disease, meaning their condition did not change. The other eleven patients’ cancer worsened.
Future Implications
Gene integration via viral vectors establish the current standard for CAR T therapy, but could soon be replaced with cheaper and more efficient CRISPR gene editing. The clinical results demonstrate that tumor-specific CAR T cells can be made and used safely; that these CRISPR-edited immune cells can recognize solid tumor masses; and that this method holds potential to be effective against drug-resistant solid tumors. While this realm of research still warrants room for improvement, especially with more uniform tumors (ex: lung tumors only), this foundation sets an excellent springboard for advancements to come.